Donanemab Faces Rejection from NHS for Widespread Use in England
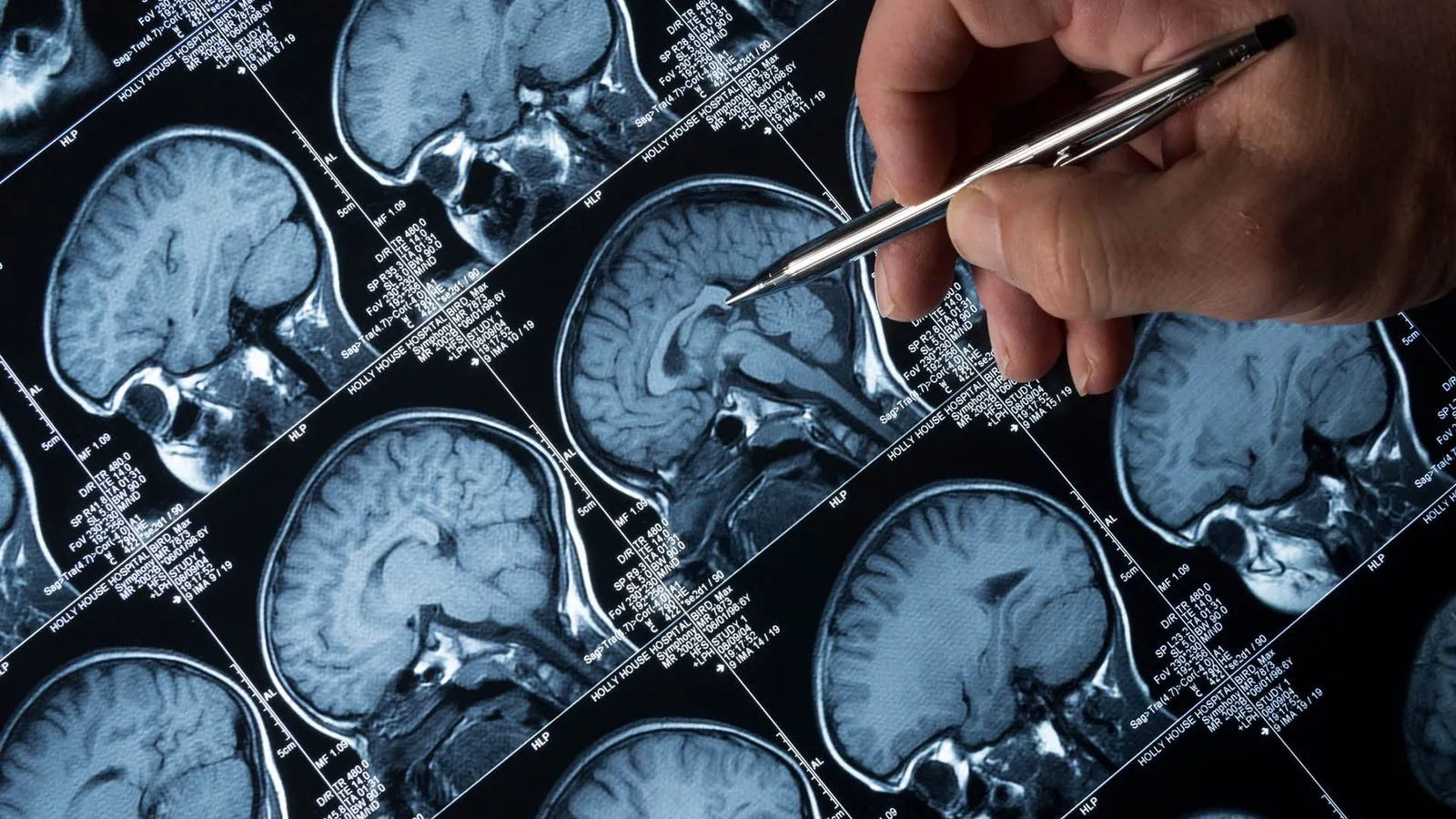
Understanding the Rejection of Donanemab
Donanemab, a promising drug aimed at treating Alzheimer's disease, has recently encountered a setback. The NHS in England has decided against its widespread use, creating ripples in the healthcare community and for patients alike.
Key Impacts of the Decision
- This rejection affects many patients who rely on innovative treatment methods.
- Health experts express concerns regarding the potential stagnation in Alzheimer's research funding.
- The future of similar drugs may be at risk, influencing pharmaceutical companies' decisions.
What Comes Next for Donanemab?
The implications of this decision are vast. As the health sector addresses the needs of Alzheimer's patients, the focus shifts towards potential next steps for clinical approval of drugs like donanemab.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.