Clearmind Biomedical Secures FDA Clearance for Neuroblade Neuroendoscopy Technology
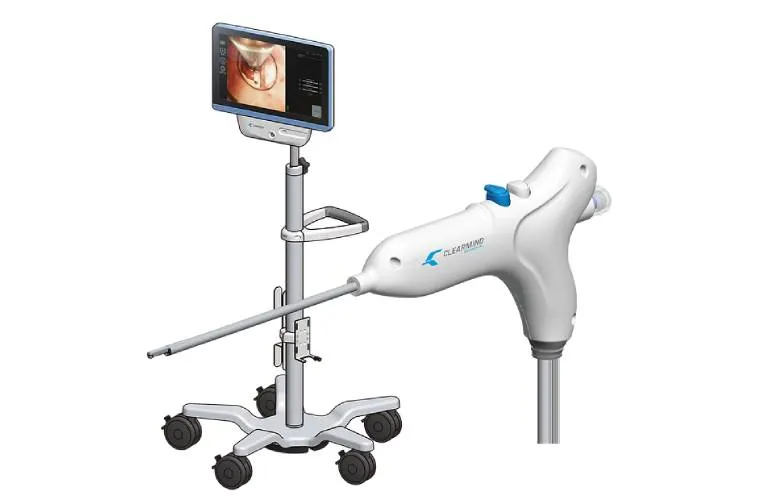
Revolutionizing Neuroendoscopy with FDA Approval
Clearmind Biomedical, a company at the forefront of medical technology, has recently received FDA 510(k) clearance for its cutting-edge Neuroblade neuroendoscopy system. This innovative solution signifies a major leap in minimally invasive procedures, providing enhanced safety and efficacy in treating various neurological disorders.
The Impacts of Neuroblade Technology
- Improved Patient Outcomes: The Neuroblade allows for more precise interventions, leading to quicker recovery times.
- Enhanced Visualization: Features advanced imaging technology, making it easier for surgeons to navigate delicate brain structures.
- Potential for Broader Applications: This technology opens doors for new treatment protocols in neurosurgery.
Conclusion and Future Directions
The FDA clearance for Neuroblade by Clearmind Biomedical not only underscores the importance of innovation in medical technology but also sets a new standard for the future of neuroendoscopy. Ongoing research and development in this area promise to further enhance surgical options available to practitioners worldwide.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.