Clearmind Biomedical Secures FDA Clearance for Neuroblade Neuroendoscopy Technology
Monday, 26 August 2024, 07:40
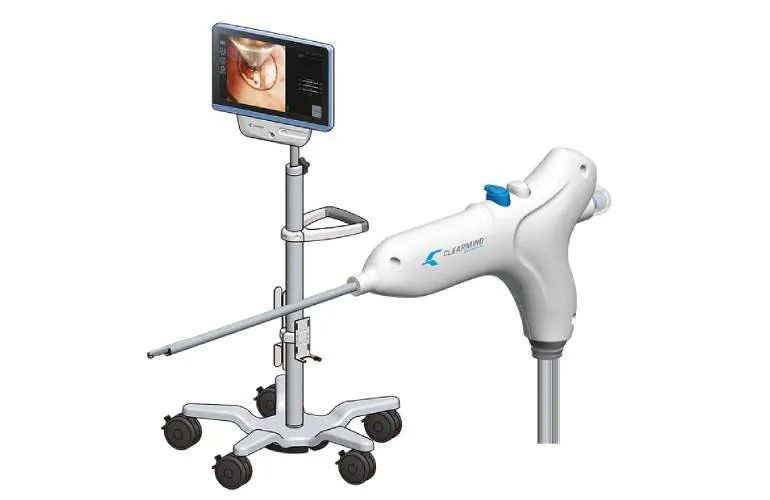
Clearmind Biomedical's Innovative Neuroendoscopy
Clearmind Biomedical has recently announced its FDA 510(k) clearance for Neuroblade, a novel neuroendoscopy system. This technology aims to streamline minimally invasive surgeries, enhancing precision in neurosurgical procedures.
Key Benefits of Neuroblade
- Minimally Invasive: Reduces recovery times and surgical risks.
- Enhanced Visualization: Offers superior imaging capabilities.
- Efficient Procedures: Aims to shorten operation durations.
As the field of neurosurgery evolves, Clearmind Biomedical stands at the forefront, pushing boundaries with Neuroblade.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.