Unveiling Secrets in Alzheimer’s Pharmaceuticals and Clinical Trials
Understanding the Hidden Dangers in Alzheimer’s Research
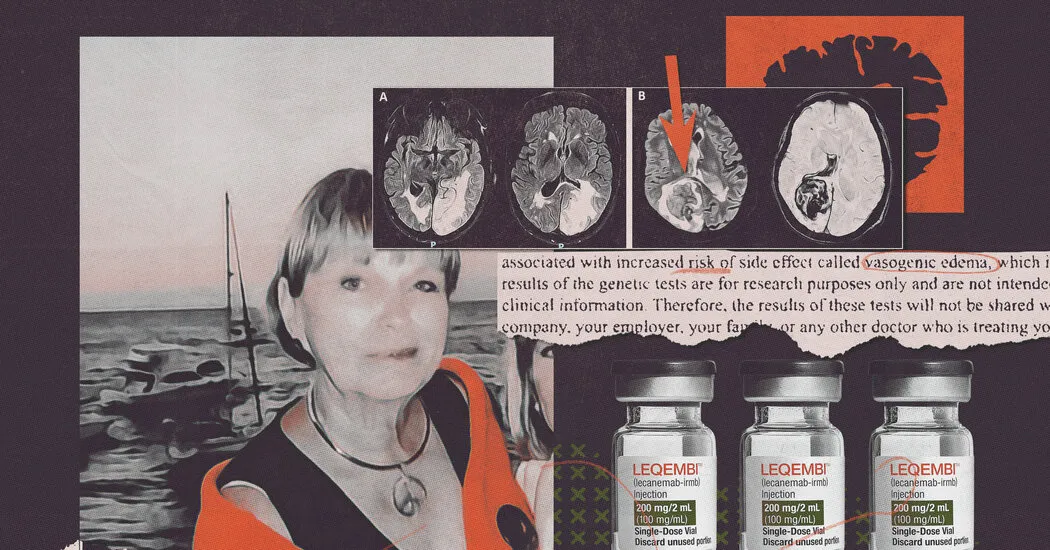
Recent advancements in Alzheimer's pharmaceuticals have sparked crucial conversations, particularly regarding committed volunteers in clinical trials. A recent study unveiled that *specific genetic tests* indicated certain patients' predispositions to severe brain injuries, risks that pharmaceutical companies kept concealed.
The Role of Genetics and Heredity
Genetics and heredity play a pivotal role in the efficacy of *prescription drugs* like Leqembi for treating Alzheimer's. With *the FDA* approving these drugs, their implications for *elderly patients* raise significant concerns.
Clinical Trials and Patient Safety
- Clinical trials show potential benefits but must disclose all risks.
- Participation in clinical research requires informed consent.
- Volunteer safety must be prioritized by pharmaceutical firms.
Implications for Retirement Communities
The implications of these findings are particularly significant for *senior citizens* and those residing in *retirement communities*. Awareness about potential health impacts from medications must be shared openly.
Conclusion: The Duty of Disclosure
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.