Rani Therapeutics Unveils New Preclinical Pharmacokinetic Findings for GLP-1 Incretin Delivery
Key Findings on GLP-1 Incretin Delivery
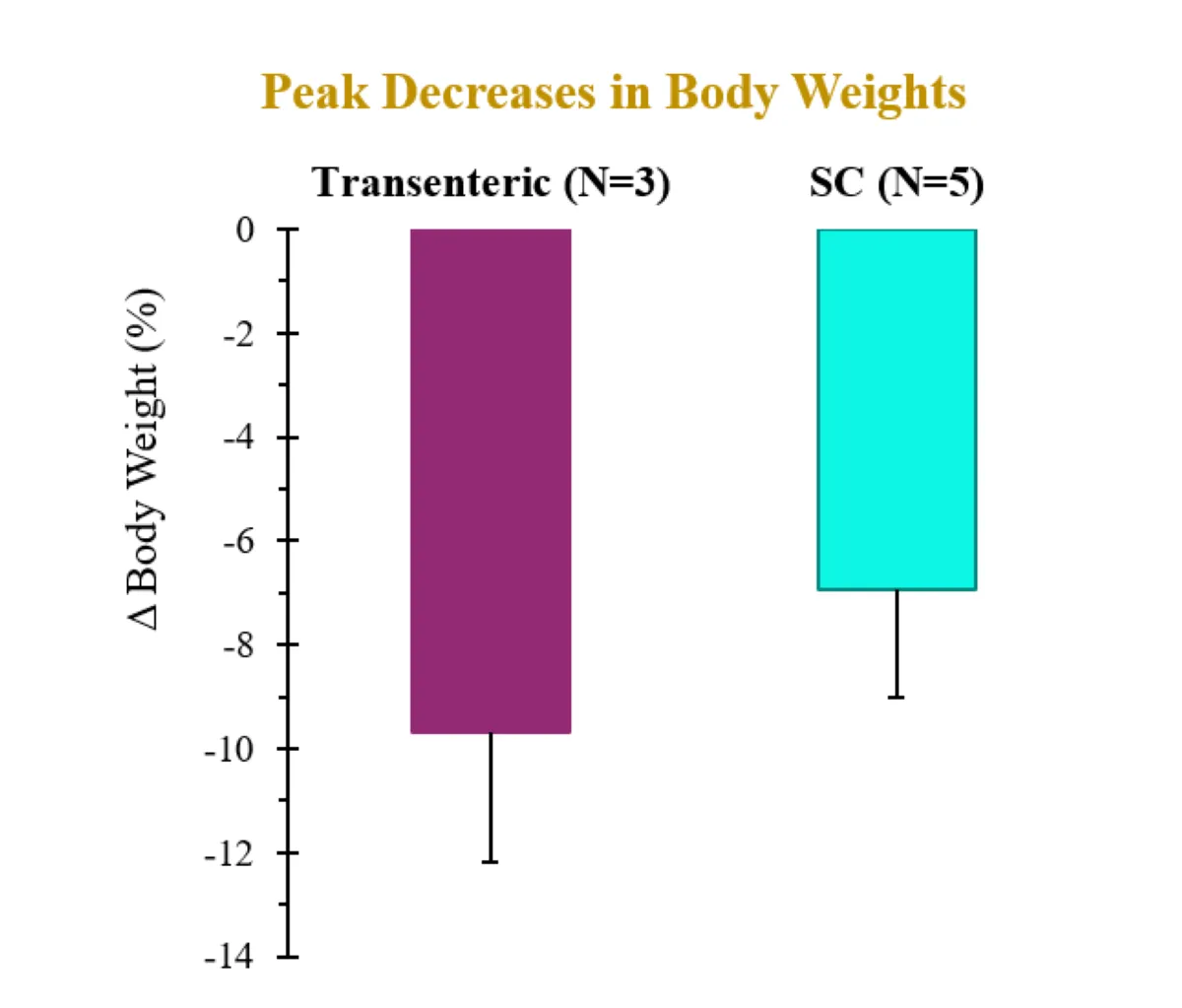
Rani Therapeutics has made waves in the biotech community by announcing new preclinical pharmacokinetic data. This data highlights the efficiency of transenteric delivery methods, particularly utilizing the RaniPill system. The results showcased 80% relative bioavailability compared to the traditional subcutaneous (SC) methods. Furthermore, the differences noted in various pharmacokinetic parameters such as AUC, C max, and T max were not statistically significant. This indicates a promising potential for enhanced drug delivery methods in the treatment landscape.
Implications for Drug Delivery Innovation
This breakthrough in transenteric delivery of GLP-1 incretin triagonist by Rani Therapeutics signals a transformative shift in how these medications might be administered in future therapies. Biotech firms are keenly watching these developments as they could pave the way for improved patient outcomes and adherence.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.