DeepWell Digital Therapeutics Achieves FDA Clearance for Stress Reduction and Hypertension
DeepWell Digital Therapeutics Achieves FDA Clearance
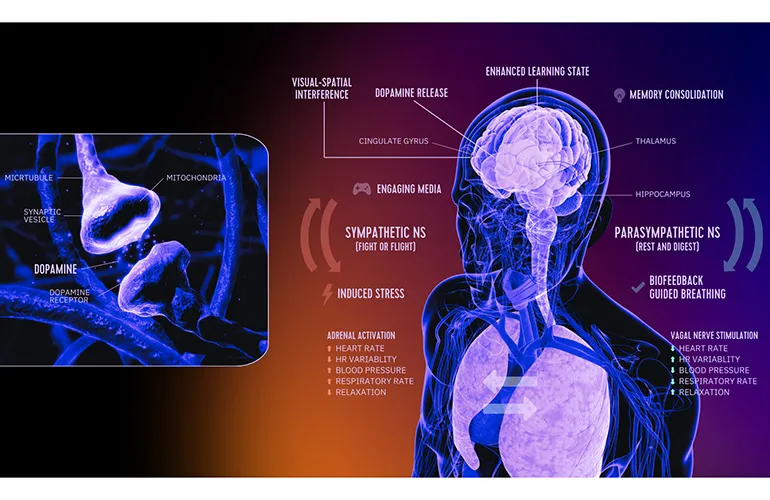
In a remarkable development, DeepWell Digital Therapeutics has received FDA clearance for its cutting-edge software development kit (SDK) aimed at facilitating digital therapeutic interventions. This SDK is specifically tailored for applications in stress reduction and managing hypertension.
Implications of FDA Approval
The FDA clearance signifies a pivotal moment in the digital health landscape, enhancing avenues for treatment through technology. The need for innovative solutions in managing conditions such as hypertension and stress is ever-growing.
- Significant enhancement in patient treatment options
- Integration of digital therapeutics in everyday health management
- Potential for better health outcomes
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.