Cook Medical IVC Filter and FDA Oversight on Medical Device Safety
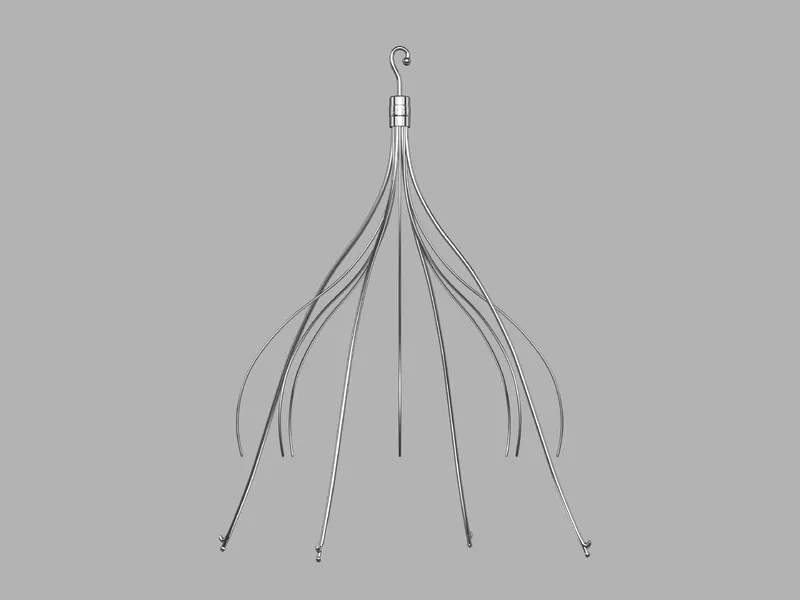
In recent developments regarding Cook Medical and its IVC filter, alarming reports of medical device malfunctions have surfaced. The FDA is under pressure to address these safety issues, particularly concerning patients who have suffered significant complications from these products.
Key points include:
- Investigation into the efficacy and safety protocols surrounding IVC filters.
- The role of the FDA in monitoring and regulating medical devices.
- Patient testimonies highlighting the potential dangers of faulty devices.
As the issue continues to unfold, it showcases the need for stringent safety measures and regulatory actions to protect patients.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.