Alzheimer's Drug Trials: Hidden Risks of Prescription Drugs
Risks of Alzheimer's Pharmaceuticals
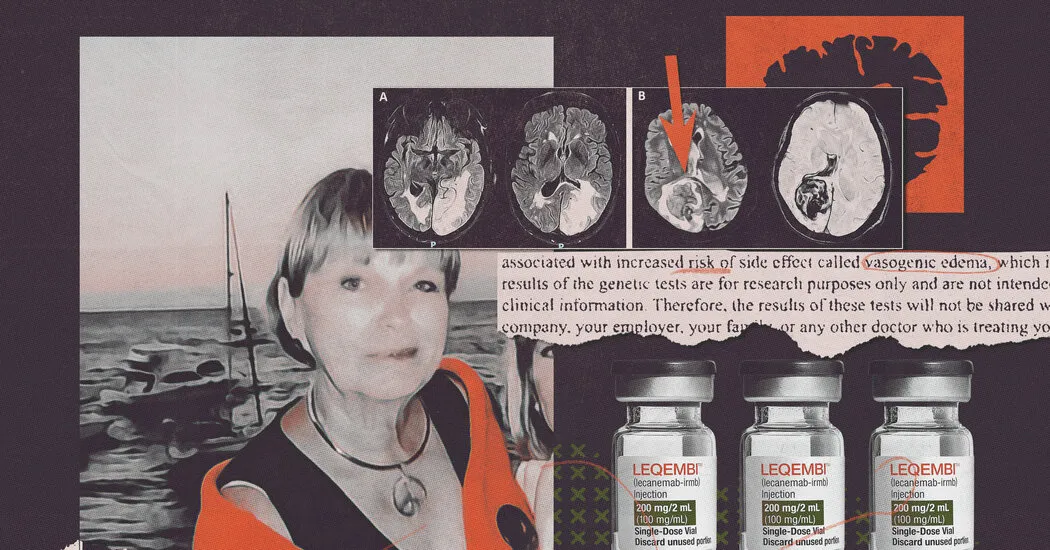
In the landscape of Alzheimer's research, clinical trials for products like Leqembi have garnered attention. However, a crucial aspect remains unaddressed: the hidden genetic risks associated with these pharmaceuticals.
Genetic Predispositions in Clinical Trials
- The Alzheimer's Assn and other organizations have called for transparency.
- Genetic tests indicated certain participants are predisposed to hemorrhagic strokes.
- Pharmaceutical companies such as Amgen, Biogen, and Eisai kept this information undisclosed.
Implications for Retirement Communities and Senior Citizens
- Informed consent remains a crucial ethical consideration.
- Awareness is vital for elderly populations considering these prescription drugs.
- Retirement communities need access to comprehensive drug information to protect residents.
For further insights, consider exploring relevant analyses from the Institute for Clinical and Economic Review and resources from the Banner Alzheimer's Institute.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.