Phase 3 Trial Results for Elevidys in Medicine Research and Health Science
Insights from the Elevidys Phase 3 Trial
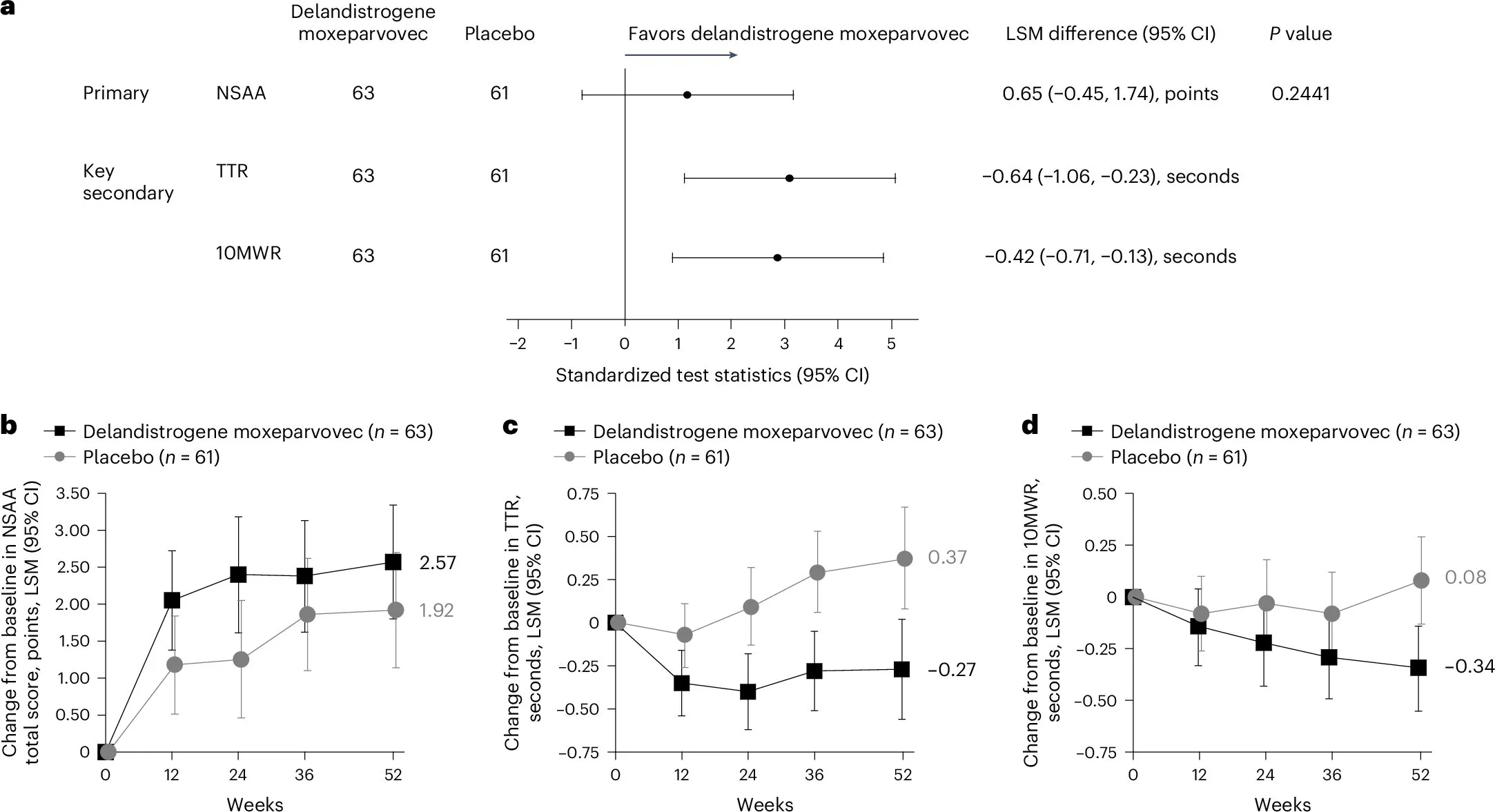
A Phase 3 clinical trial investigating Elevidys (delandistrogene moxeparvovec), a gene therapy for Duchenne muscular dystrophy, did not achieve its primary endpoint, according to findings published in the latest medicine research.
Key Findings
- Elevidys Treatment Costs: Priced at $3.2 million per dose, Elevidys raises questions about cost-effectiveness in health research.
- Patient Responses: The trial highlighted variable patient responses, raising concerns about the therapy's overall effectiveness.
- Future Research Directions: The trial's failure prompts reassessment of methodologies in medicine science related to gene therapies.
Implications for Health Science
This outcome not only affects the future of Elevidys but also poses challenges for other healthcare investments into gene therapies targeting rare diseases.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.