Healthcare Advancements: Almirall Completes EU Regulatory Procedure for Jublia
Healthcare Innovations: Almirall's Progress
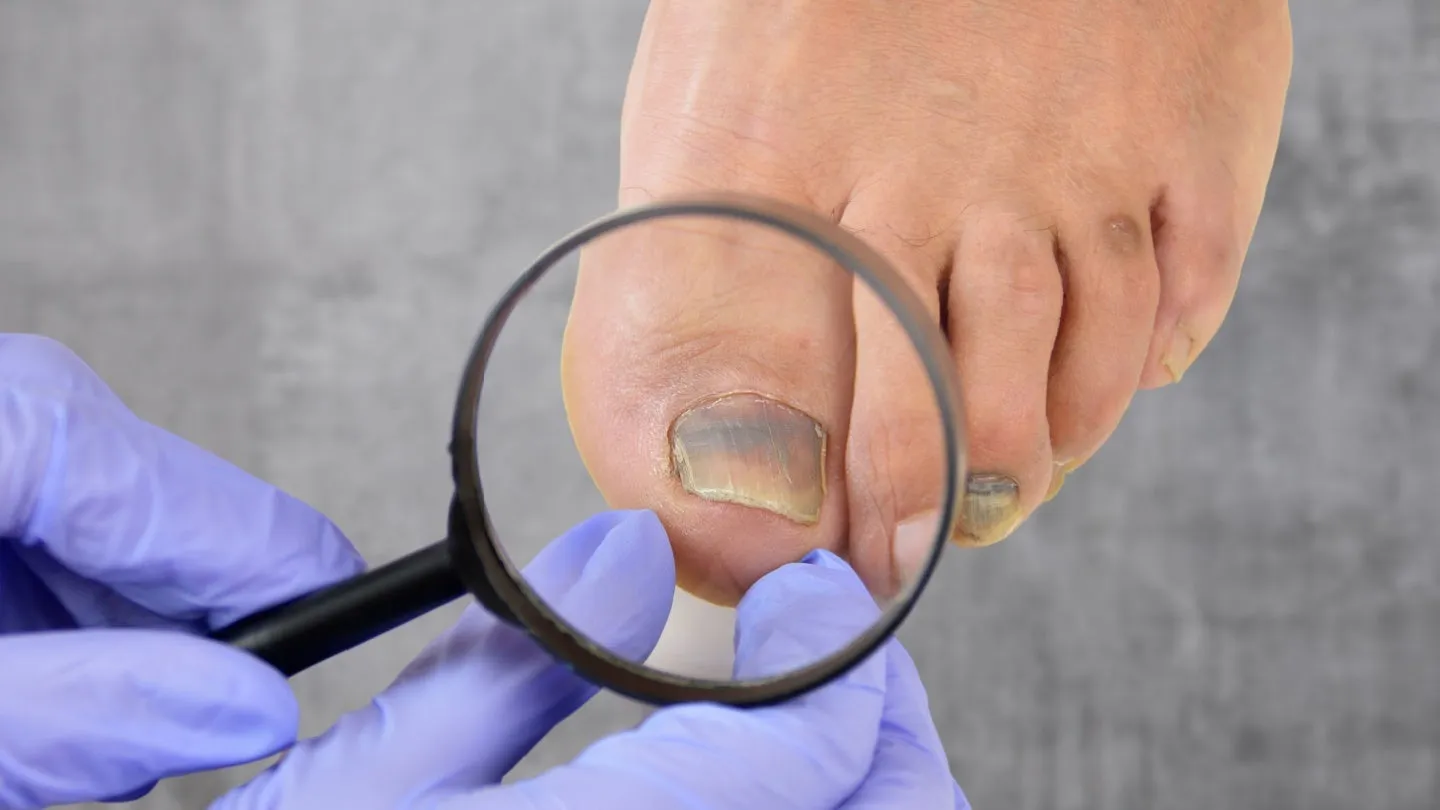
In a significant move for healthcare, Almirall has successfully completed the decentralised regulatory procedure in Europe for Jublia, a topical solution utilizing efinaconazole. This treatment specifically targets onychomycosis, a fungal infection of the nails that affects millions.
Significance of Jublia
- Targeted Treatment: Jublia represents a targeted approach to treat nail fungus, which can often lead to discomfort and embarrassment.
- With its approval, there is potential for significant improvement in patient quality of life.
- This regulatory success is a testament to Almirall's commitment to advancing medical treatment.
Next Steps in Healthcare
- Healthcare providers will need to familiarize themselves with the administration of Jublia.
- Monitoring outcomes post-approval will help gauge the effectiveness of the treatment.
As healthcare continues to evolve, innovations like Jublia pave the way for better and more accessible solutions in the treatment landscape.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.