BioIntelliSense Achieves FDA Approval for Innovative BioButton Wearable Monitor
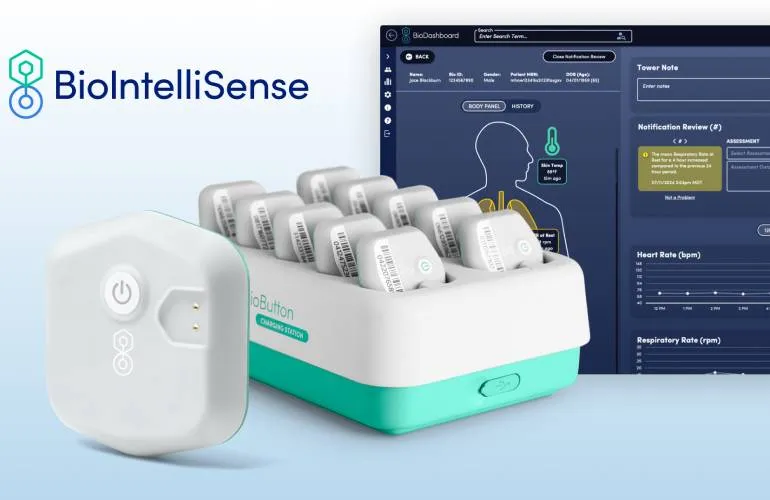
FDA Approval of BioIntelliSense's BioButton Monitor
In a significant development for wearable medical technology, BioIntelliSense announced today that it secured FDA clearance for its rechargeable BioButton multi-patient wearable monitor. This revolutionary device, designed to monitor various patients simultaneously, integrates with the BioDashboard system, enabling efficient and effective health management.
Key Features of the BioButton
- Multi-patient monitoring capabilities
- Rechargeable and long-lasting battery life
- Integration with advanced analytics through the BioDashboard
This approval is expected to enhance healthcare delivery by providing healthcare professionals with real-time health data, improving patient outcomes significantly.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.