Dermatology: LEO Pharma's NDA Submission for Enstilar to Treat Plaque Psoriasis
LEO Pharma's Submission
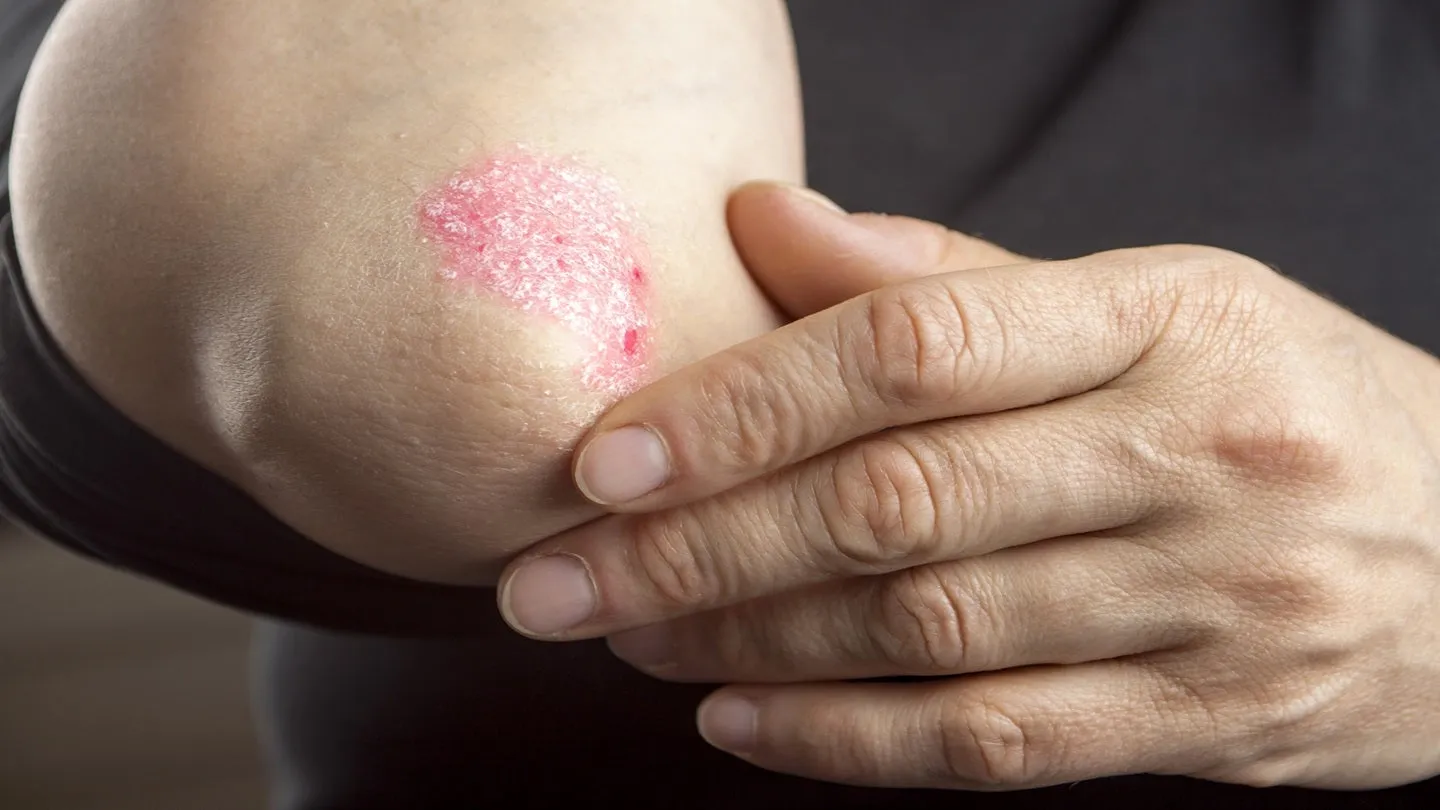
LEO Pharma has officially submitted a New Drug Application (NDA) to China's National Medical Products Administration for Enstilar, which is poised to be a game-changer in the treatment of plaque psoriasis.
Significance of Enstilar
Enstilar offers a unique combination of calcipotriene and betamethasone dipropionate, which works synergistically to effectively manage the symptoms of this severe skin condition.
Impact on Dermatology
- Enhances current treatment options for plaque psoriasis
- Aims to improve patient outcomes and quality of life
- Potential to reduce healthcare costs associated with chronic dermatological conditions
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.