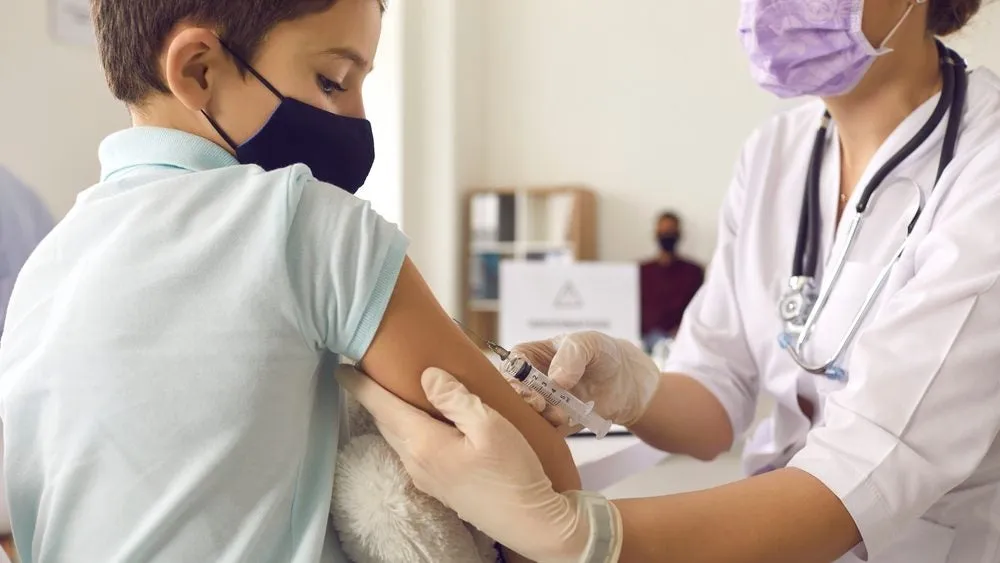
Covid-19 Trial: Controversy Surrounds Moderna's £1,500 Incentive for Children
Ethical Concerns in Covid-19 Trials
Recently, reports surfaced regarding Moderna's practice of offering children a monetary incentive of £1,500 to take part in its Phase III NextCOVE trial for Covid-19. Such actions have prompted significant criticism from various sectors of the medical community.
Background of the Trial
The NextCOVE trial involves testing the efficacy and safety of a new Covid-19 vaccine. The decision to incentivize children financially has ignited debates about ethical standards in clinical trials.
Implications for Future Research
The backlash against this approach underlines the need for stricter governance in the recruitment of minors for medical research. Proponents argue that fair compensation is essential, while critics question the morality of incentivizing vulnerable populations.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.