Clinical Development and Patient Centricity in Sickle Cell Disease as Pfizer Withdraws Oxbryta
Overview of Pfizer's Withdrawal of Oxbryta
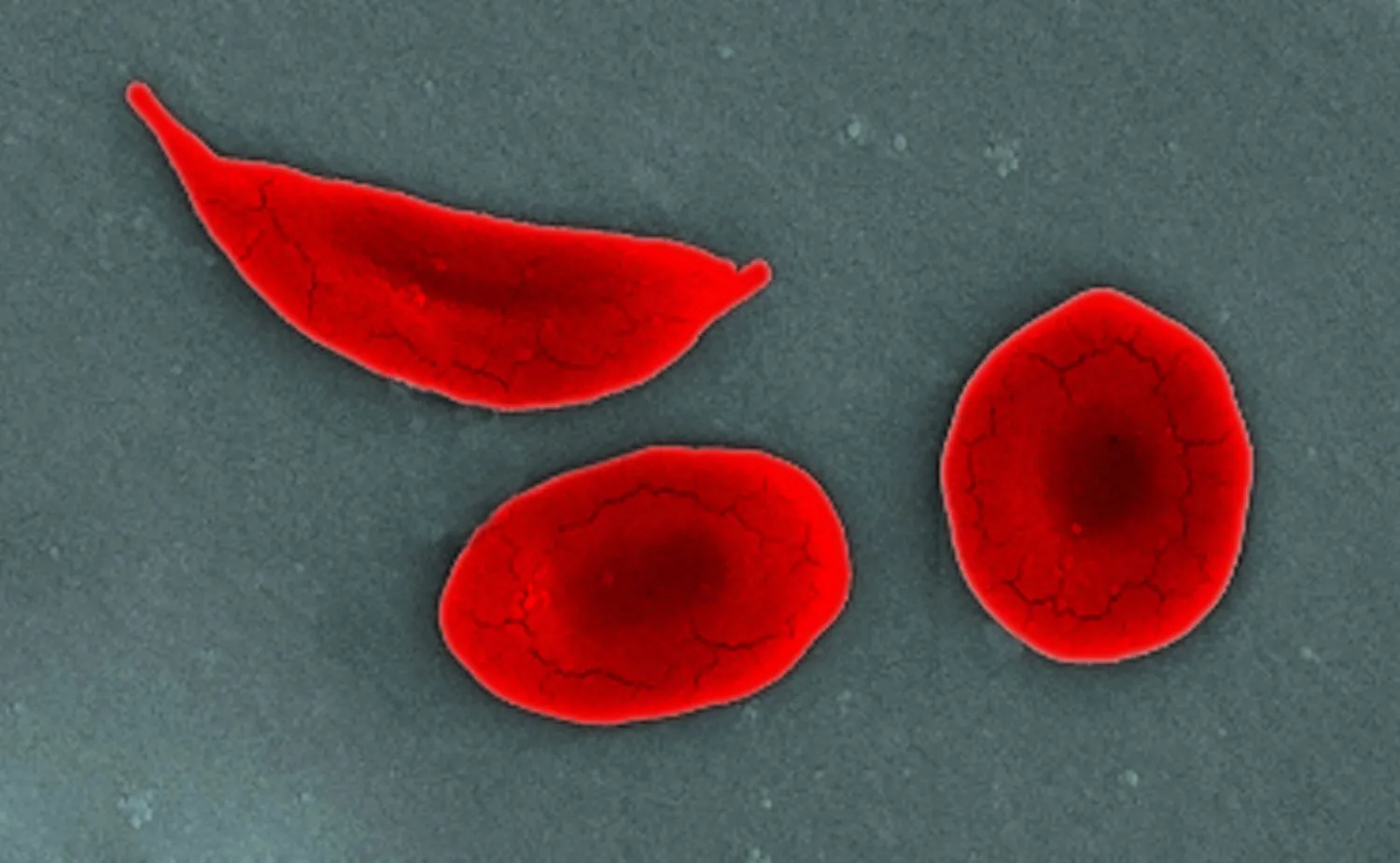
Clinical development efforts have faced a major hurdle with Pfizer's recent announcement regarding Oxbryta, an oral treatment for sickle cell disease. The decision to voluntarily withdraw the drug from the market and halt ongoing Phase I-II and Phase III-IV clinical trials is largely due to emerging safety concerns. Reports indicate an elevated risk of severe adverse effects among patients, raising significant concerns regarding patient centricity in clinical trials.
Implications for the Pharmaceutical Industry
This withdrawal not only impacts the FDA approval process but also reflects a deeper conversation about the responsibilities of pharmaceutical companies in safeguarding patient health during clinical development. As Oxbryta is pulled from the market, the industry must evaluate how to ensure patient safety while pushing forward with innovative treatments.
Concluding Thoughts on Patient Safety
The situation with Oxbryta serves as a critical reminder of the fundamental need for rigorous safety evaluations throughout the drug development process. Stakeholders in healthcare must collaborate more effectively to align with patient centricity principles, ensuring the highest standards of safety and efficacy in the treatment of sickle cell disease.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.