Science in the Pharmaceutical Industry: Addressing Health Medication Shortages in France
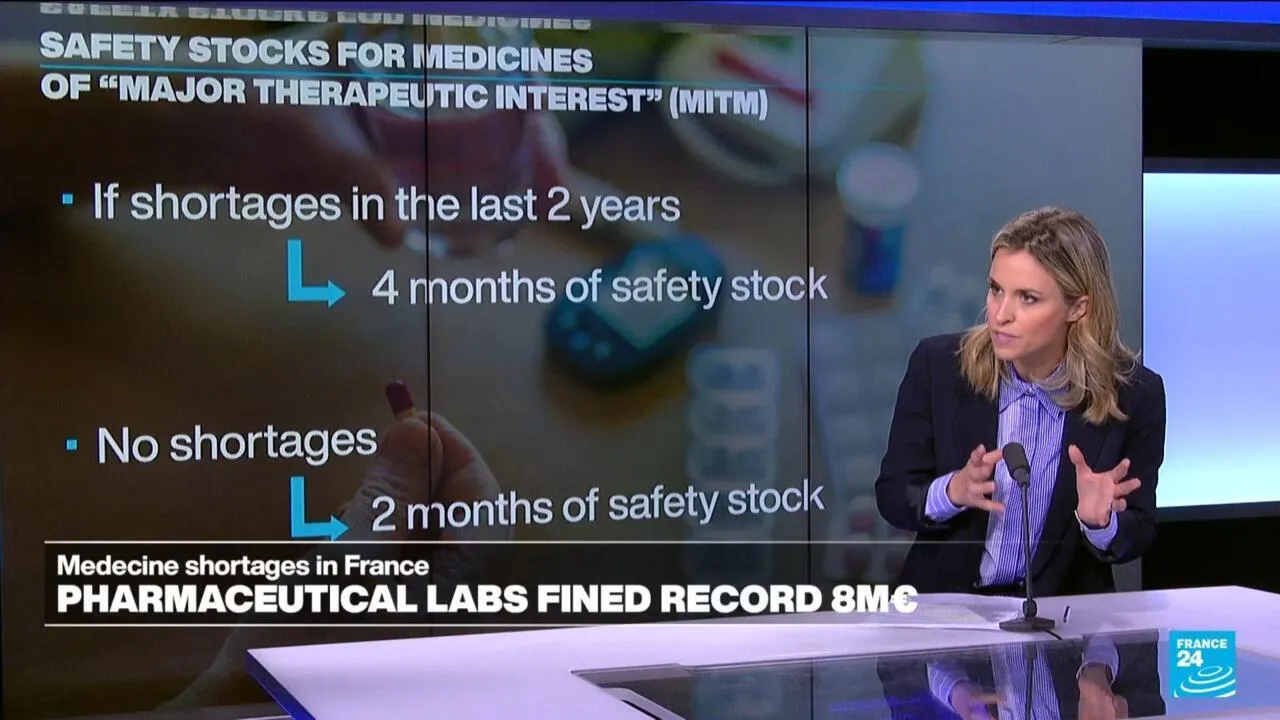
The French Medicines Agency has levied unprecedented fines against 11 pharmaceutical companies for failing to adhere to the required minimum stocks of medications deemed "of major therapeutic interest." These penalties serve as a vital reminder of the importance of maintaining adequate supplies to safeguard public health.
The Root Causes of Medication Shortages
Medication shortages have become a pressing concern globally. In France, these shortages are attributed to several factors:
- Supply chain disruptions
- Increased demand for certain medications
- Manufacturing issues
The Regulatory Response
In response to these challenges, the French Medicines Agency has implemented stringent regulations. Companies are now mandated to ensure that their inventories align with safety stock requirements for critical medications.
Looking Forward: Ensuring Health and Safety
The pharmaceutical industry must prioritize compliance with health regulations to prevent future shortages. Building a resilient supply chain is essential for safeguarding public health.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.