Rinvoq Shows Promising Results for Atopic Dermatitis in Head and Neck Areas
Rinvoq Shows Promising Results for Atopic Dermatitis in Head and Neck Areas
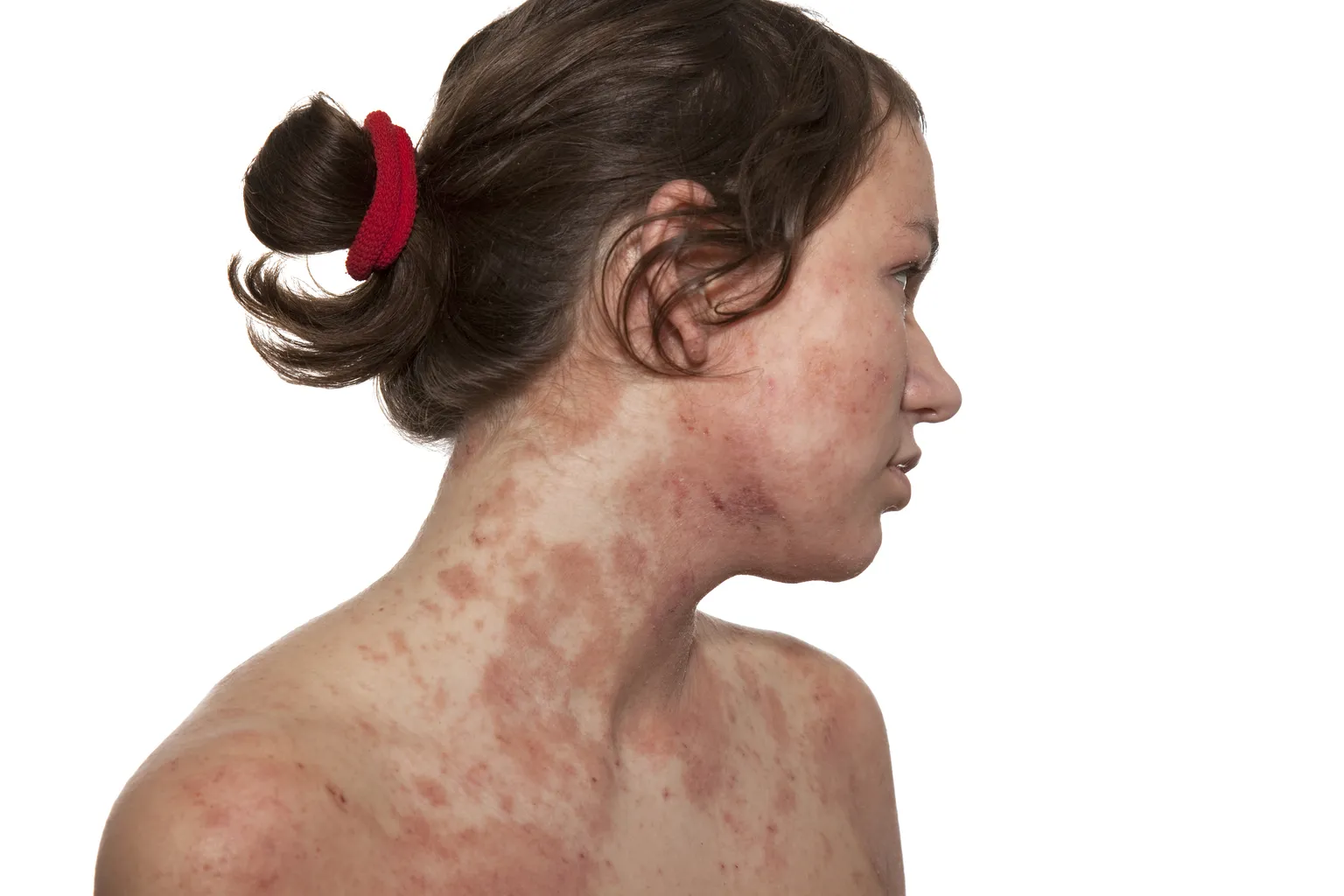
AbbVie’s Rinvoq has emerged as an effective treatment for moderate-to-severe atopic dermatitis affecting the head and neck regions. Clinical studies demonstrate impressive outcomes, showing that patients achieved significant skin clearance alongside improved quality of life metrics.
Clinical Insights
In a study involving participants suffering from atopic dermatitis, results indicated that after 16 weeks of treatment with Rinvoq, many experienced marked improvements.
- 76% of patients achieved skin clearance.
- Quality of life assessments showed substantial enhancements in daily living activities.
- Adverse effects were minimal, reinforcing the safety profile of Rinvoq.
Implications for Treatment
The approval of Rinvoq by AbbVie represents a major step forward in the treatment of challenging dermatological conditions. With consistent results, healthcare providers are encouraged to consider this medication as a viable option for their patients.
Next Steps for Patients
Patients are advised to consult their healthcare providers to explore suitability and potential integration of Rinvoq into their treatment plans.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.