Approvals in Drug Development: The EC Authorises LEO Pharma's Anzupgo Cream
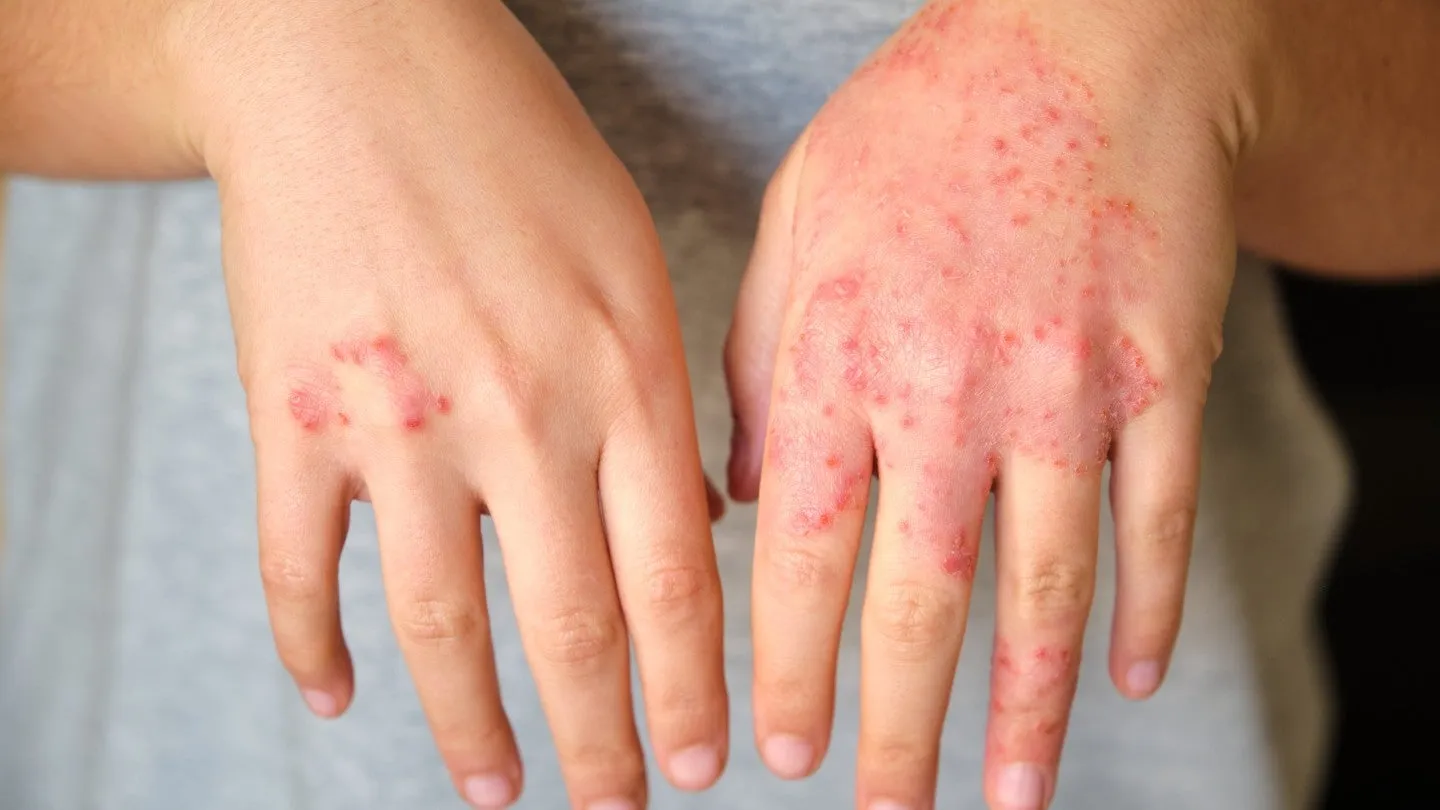
Overview of Anzupgo Cream
In a significant move within drug development, the European Commission (EC) has officially granted marketing authorisation to LEO Pharma's Anzupgo cream. This therapeutic cream is specifically aimed at treating adults afflicted with moderate to severe chronic hand eczema, addressing a pressing need in the dermatological community.
Importance of this Approval
This exciting development underlines the importance of timely approvals in drug development. By securing this status, LEO Pharma enhances treatment accessibility for patients grappling with chronic skin conditions.
Future Implications
- Enhanced Patient Care: With the launch of Anzupgo cream, patient outcomes in managing eczema are expected to improve.
- Sparking Innovations: This approval is anticipated to inspire further research and innovation in therapeutic treatments.
- Market Entry: The introduction of Anzupgo cream marks another significant entry in the dermatological market.
For more details about this groundbreaking approval and its implications, please consult the source for comprehensive insights.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.