At-Home Flu 'Shot': A New Era in Self-Administered Vaccination
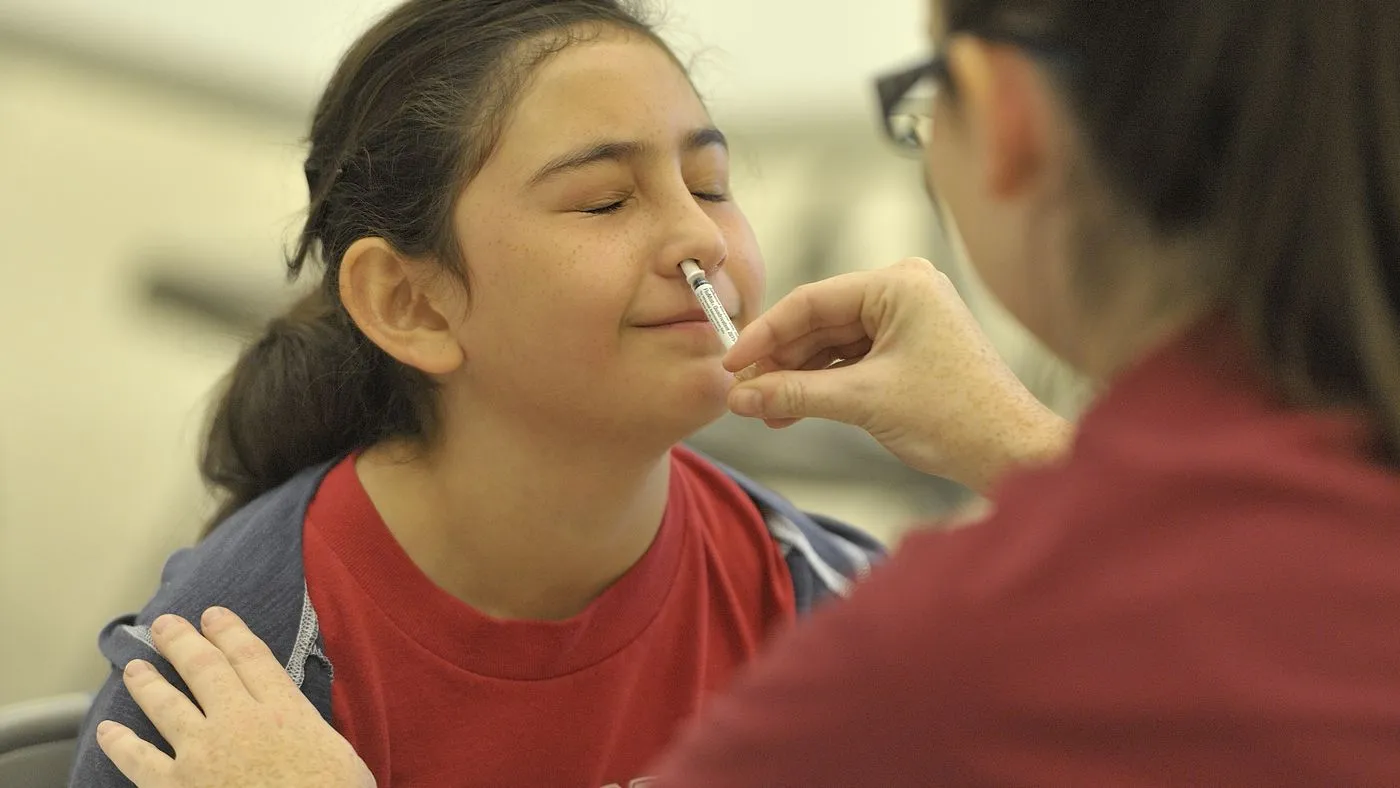
Introducing At-Home Flu 'Shot'
The Food and Drug Administration has approved a nasally administered influenza immunization treatment that can be taken at home. FluMist — a nasal spray vaccine initially developed by AstraZeneca — will still require a prescription. Set to be available via an online pharmacy next year, those interested will need to complete a questionnaire on the FlueMist Home website. Upon approval by a pharmacist, the nasal spray will be shipped directly to the customer’s door.
The current out-of-pocket cost is approximately $35 to $45 per dose, depending on the availability of insurance coverage. The Centers for Disease Control and Prevention notes that FluMist exhibits a similar efficacy to traditional flu shots.
Convenience and Accessibility
- FluMist is suitable for individuals aged 2 to 49.
- Children under 18 require parental or caregiver administration.
- This nasal spray option could appeal to those hesitant about injections or those facing travel challenges for flu immunizations.
FDA Director Dr. Peter Marks stated, “Today’s approval of the first influenza vaccine for self- or caregiver-administration provides a new option for receiving a safe and effective seasonal influenza vaccine potentially with greater convenience, flexibility, and accessibility for individuals and families.” The World Health Organization informs that around a billion cases of seasonal influenza occur annually, leading to 290,000 to 650,000 respiratory deaths each year.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.