FDA Approves First At-Home Flu Vaccine: FluMist Nasal Spray
Friday, 20 September 2024, 11:30
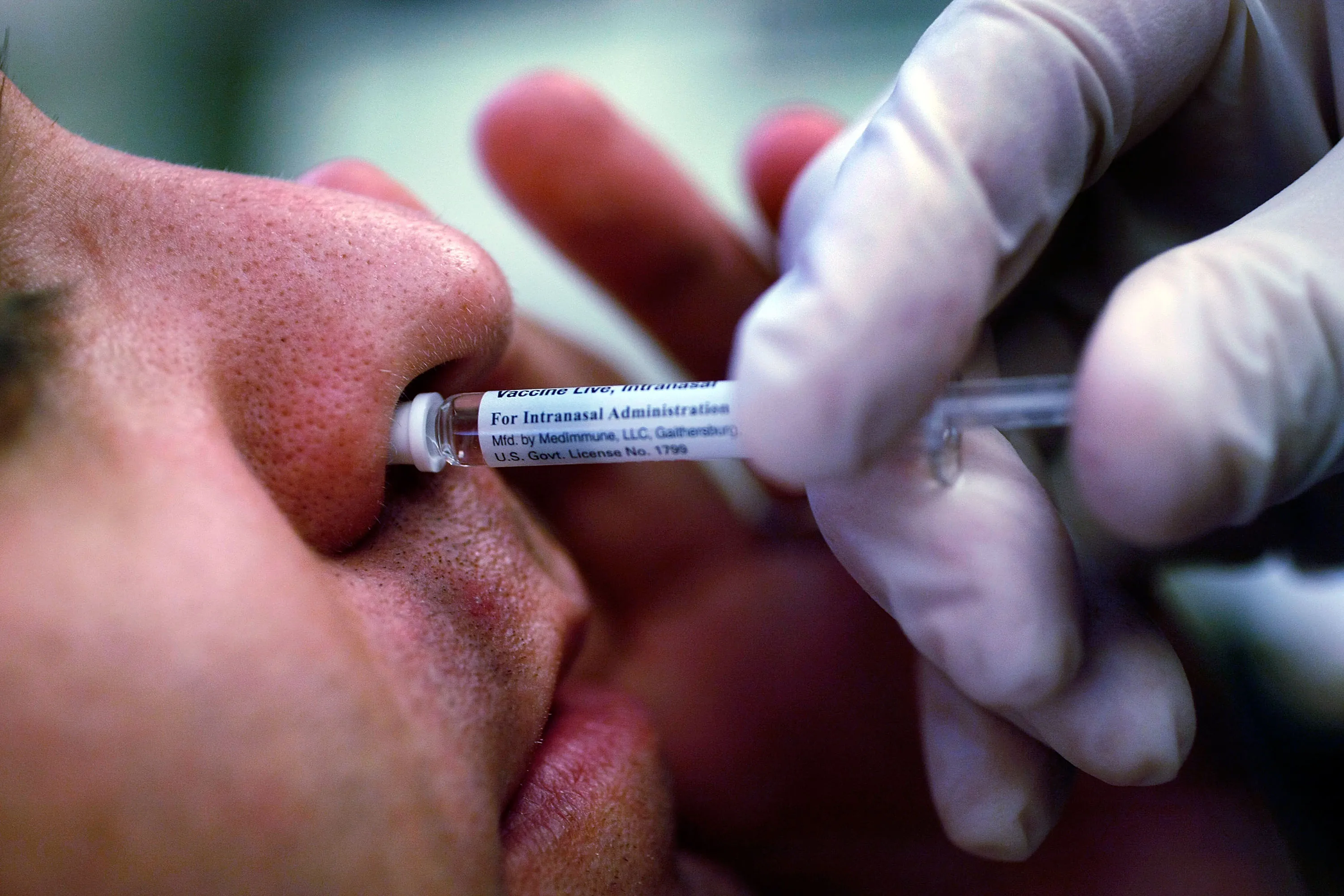
Revolutionary Flu Prevention
FluMist is now recognized as the first flu vaccine approved for at-home use in the U.S. Standing out from traditional flu vaccines, this intranasal administration method caters to individuals between the ages of 2 and 49.
Key Benefits of FluMist
- Convenient nasal spray application.
- Applicable for a broader age demographic.
- Enhanced compliance due to ease of use.
This FDA approval signifies a critical milestone in accessibility to flu vaccines, addressing public health needs with innovative solutions.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.