FDA Approves First Flu Vaccine for At-Home Use: A Revolutionary Step in Influenza Prevention
Friday, 20 September 2024, 12:31
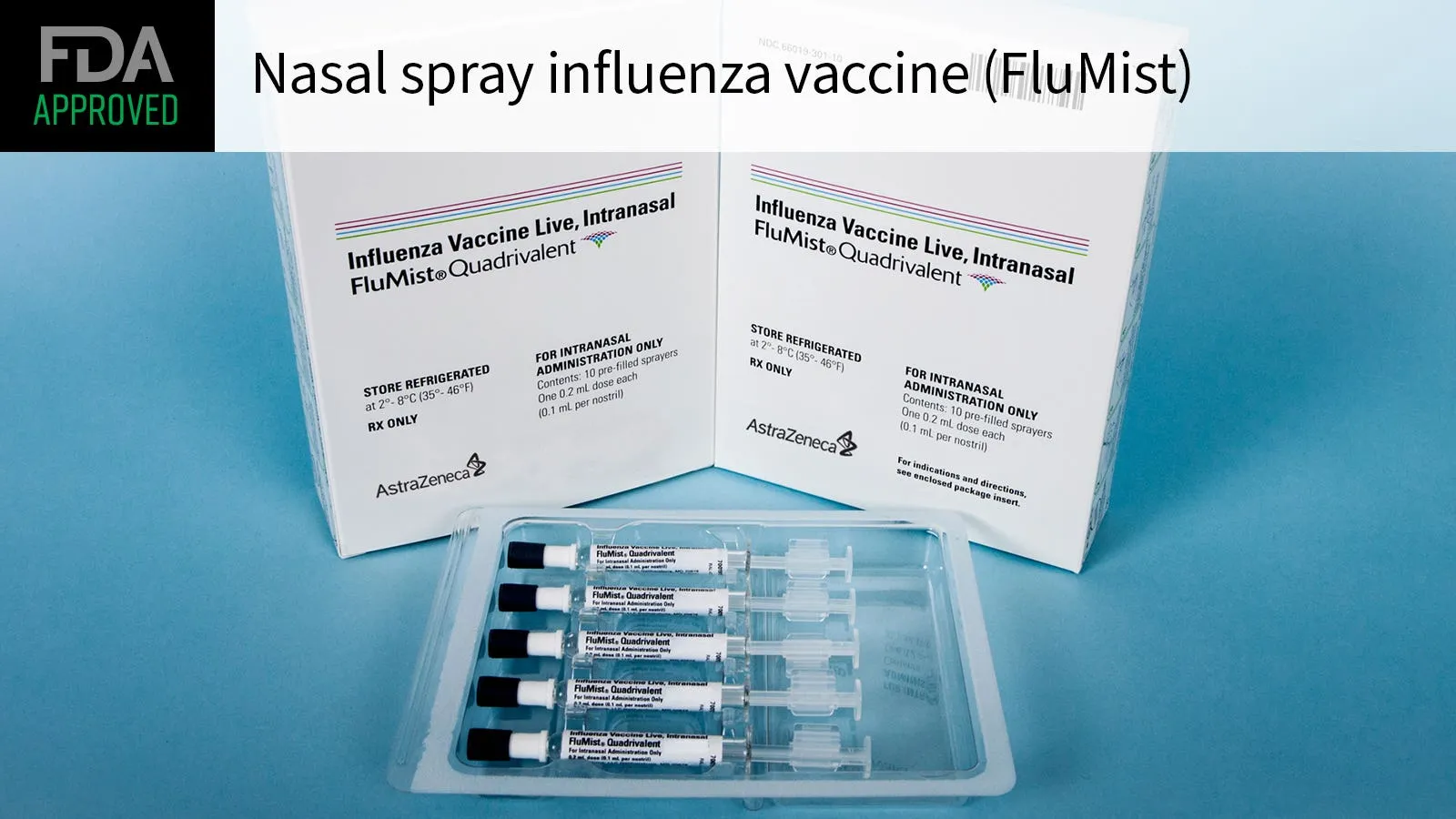
FDA Approval of FluMist
The FDA officially announced the approval of FluMist, marking it as the first influenza vaccine that can be administered at home. This groundbreaking decision eliminates the necessity for healthcare provider involvement, enhancing accessibility for individuals seeking protection against influenza.
Implications for Public Health
- Increased Vaccination Rates: With at-home administration, more people can easily receive their flu shots, potentially increasing vaccination coverage.
- Empowering Patients: This change enables individuals to take control of their health by allowing families to administer the vaccine without the need for a clinic visit.
- Enhancing Community Health: By improving access, community health outcomes may significantly benefit during flu seasons.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.