FDA Approves First Self-Administered Flu Vaccine Spray for Flu Season
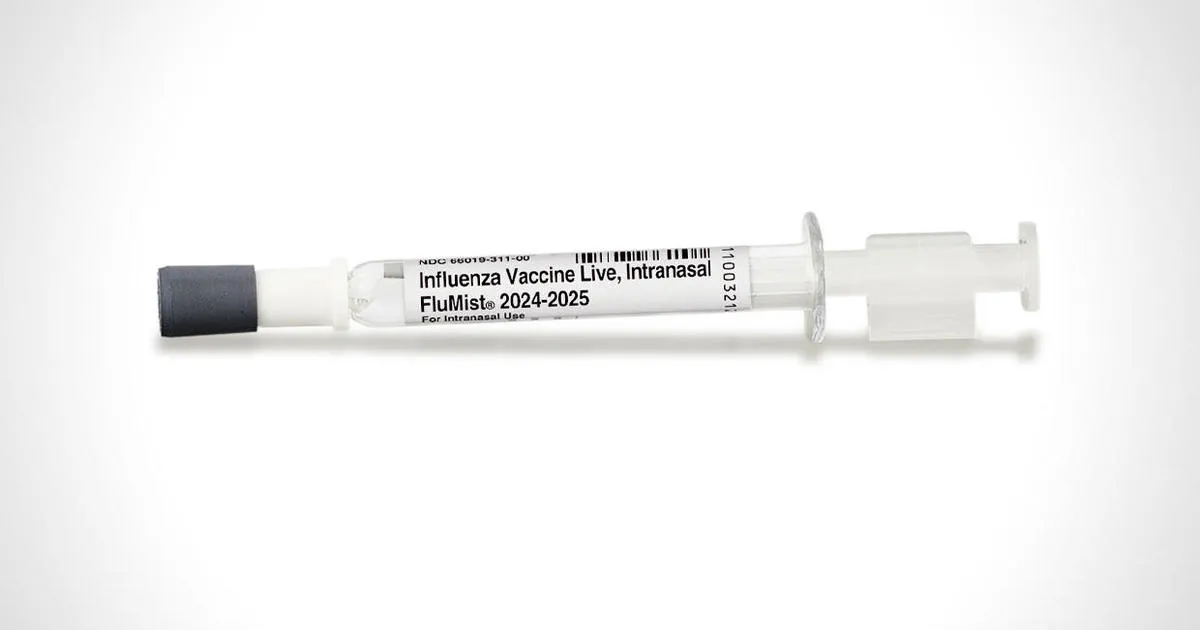
FDA's Approval of Self-Administered Flu Vaccine Spray
The Food and Drug Administration (FDA) has recently approved the use of FluMist, a nasal spray vaccine that can be administered by individuals themselves. This groundbreaking decision aims to increase vaccination accessibility during flu season.
Key Benefits of the Self-Administered Vaccine
- Improved Accessibility: Individuals can now vaccinate without needing to visit a healthcare provider.
- Potential Increase in Vaccination Rates: The convenience of self-administration is expected to encourage higher uptake of flu vaccinations.
- Enhanced Public Health Protection: Wider vaccine distribution can help mitigate flu outbreaks.
Flu Season Preparedness
The approval comes as flu season approaches, presenting an ongoing challenge in public health. The FDA's commitment to improving vaccination strategies reflects the urgency of addressing seasonal health threats.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.