Tandem Diabetes Advances with EU Clearance of Insulin Pump for Lilly Insulin
Tandem Diabetes Achieves EU Approval
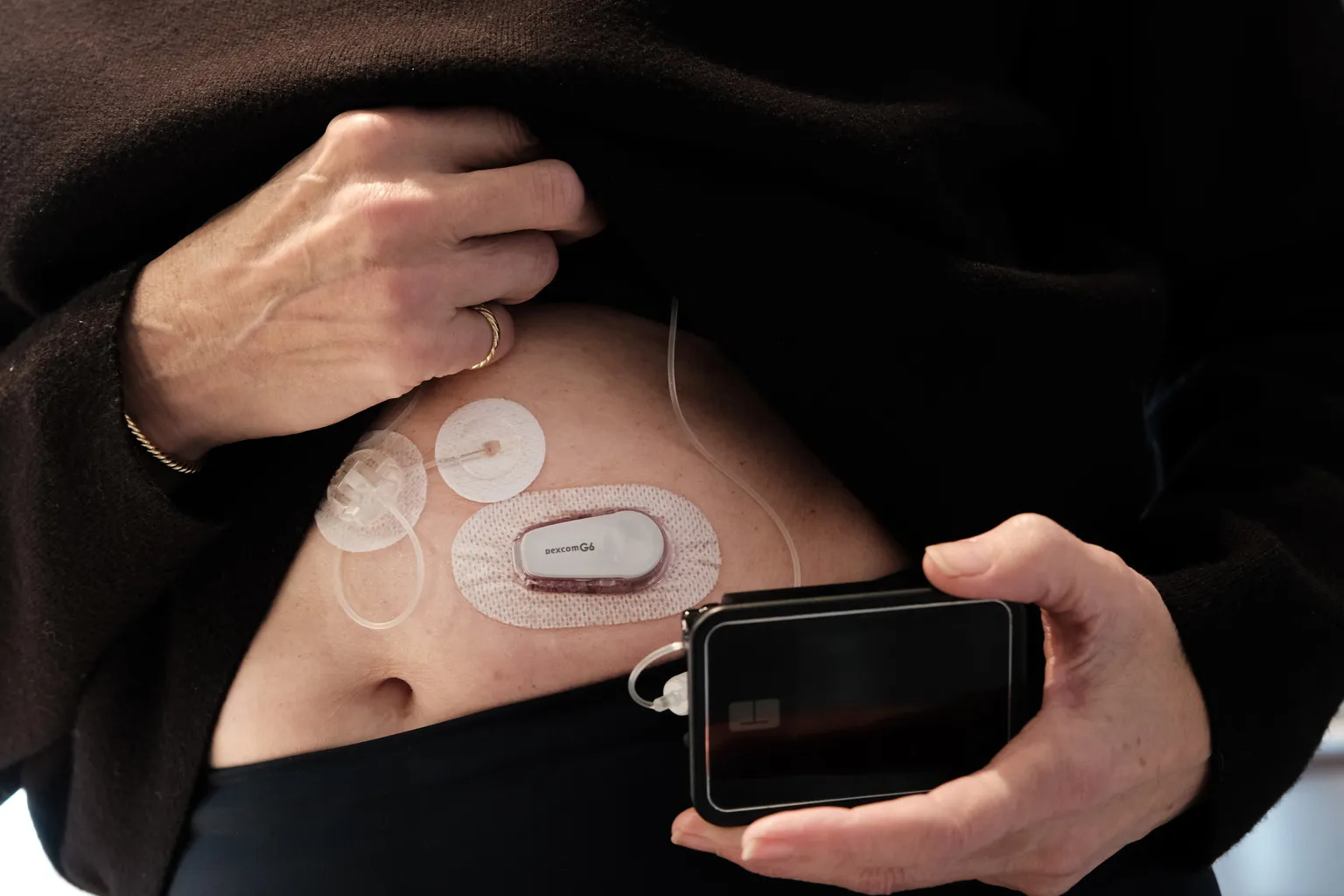
Tandem Diabetes Care has made headlines after its t:slim X2 insulin pump received clearance for use with Lyumjev, a Lilly insulin product, in the European Union. This approval is expected to enhance the management capabilities for patients who rely on insulin therapy.
Impact on Diabetes Management
- The integration of the t:slim X2 insulin pump with Lilly's Lyumjev can optimize insulin delivery.
- Patients may experience improved blood sugar control, reducing the risk of potential complications.
- This advancement highlights Tandem Diabetes's commitment to enhancing patient quality of life.
Market Reaction
Tandem Diabetes has shown positive market performance, with shares rising by approximately 3% following the announcement of this clearance. Investors are optimistic about the growing potential of Tandem’s products in the competitive diabetes technology market.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.