FDA Approves Ebglyss: A Breakthrough in Medicine Research for Atopic Dermatitis
FDA Approval Highlights
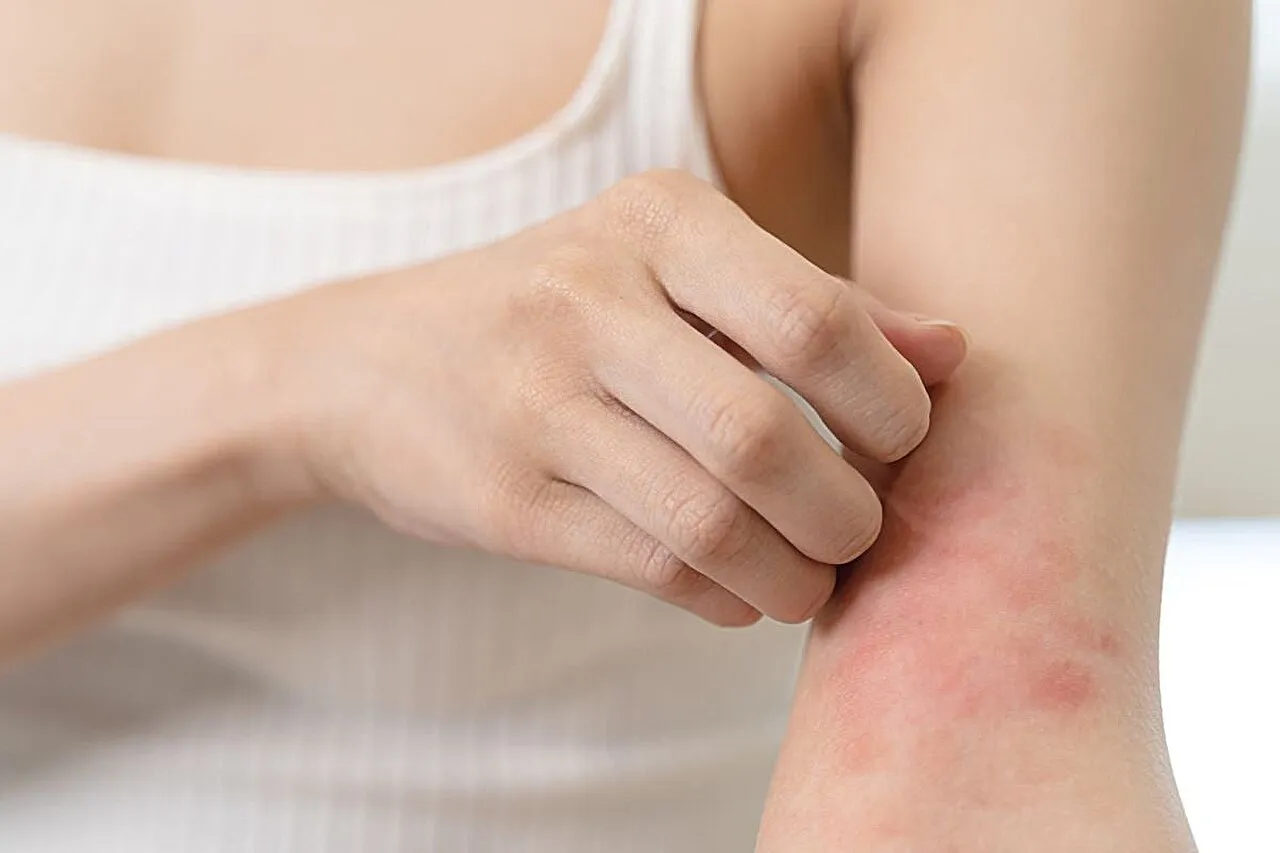
The U.S. Food and Drug Administration has granted approval for Ebglyss (lebrikizumab-lbkz), marking a significant milestone in medicine research. This treatment is indicated for individuals 12 years and older who face challenges with moderate-to-severe atopic dermatitis.
Benefits and Impact
- Ebglyss offers a new therapeutic option for patients.
- It addresses the pressing need for effective treatments in health research.
- Potential for positive changes in patient management and satisfaction.
Future Directions in Health Science
This development showcases the ongoing progress in medicine science and the commitment to advancing patient care. Ongoing studies will be crucial to further evaluate long-term efficacy and safety.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.