Neuralink's Innovative 'Blindsight' Implant: Key FDA Designation
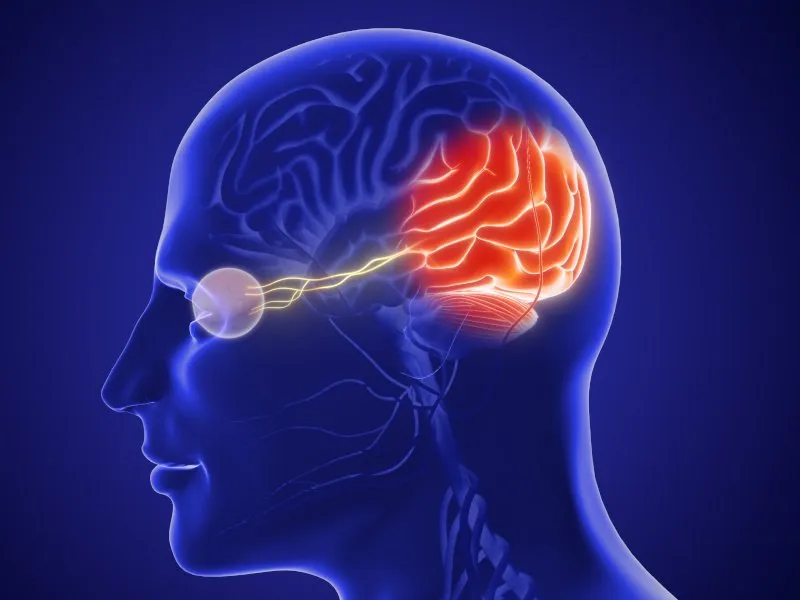
Neuralink's 'Blindsight' Implant Overview
Neuralink, the brainchild of Elon Musk, has developed an innovative device known as the 'blindsight' implant. This device recently received a pivotal FDA designation. The aim is bold: to restore vision for those who have been blind from birth or have lost their eyesight due to severe optic nerve injuries.
Implications of the FDA Designation
The FDA designation is a significant milestone, granting Neuralink the ability to advance its research and trials. The potential impact on patients dealing with profound vision loss is enormous.
- Innovative technology harnessing neural connections
- Promising initial research findings
- Potential for wide-ranging applications in ophthalmology
Future Prospects
As Neuralink progresses, updates will be essential for medical professionals and the public alike. This innovation represents a critical frontier in the interplay between neuroscience and restorative technologies.
For more details, a comprehensive examination of the implications and ongoing studies can be found at the original source.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.