Breast Cancer Screening: Exploring the Impact of New FDA Requirements on Mammograms
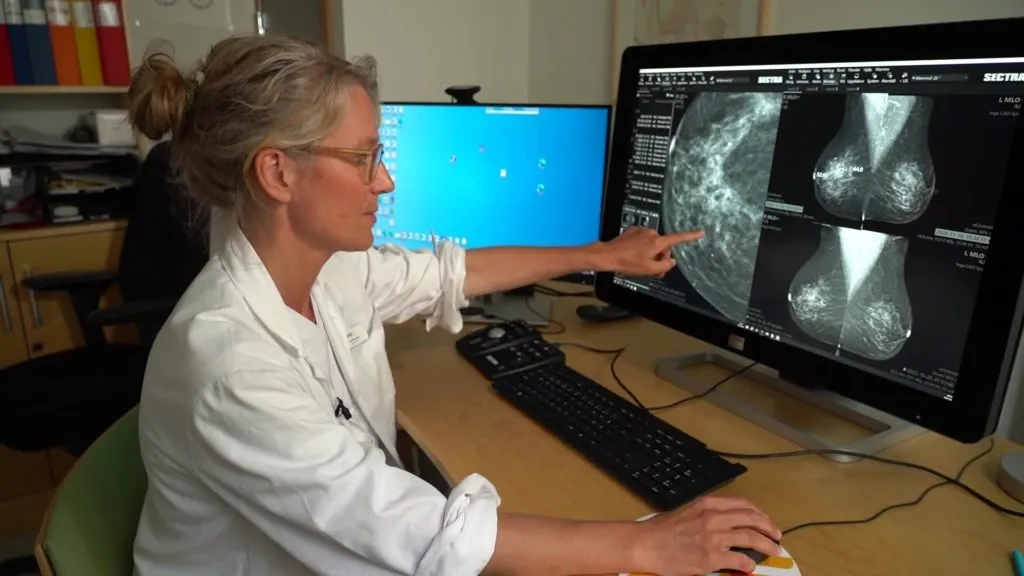
Understanding the New FDA Requirements for Mammograms
Breast cancer screening is essential for early detection. According to recent guidelines, about 1 in 8 women may be diagnosed with this disease in their lifetime. The new FDA requirement focuses on enhancing access to mammograms, the cornerstone of effective breast cancer detection.
Why Mammograms Matter
- Early Detection: Routine screenings can help identify tumors before they progress.
- Guidelines by Age: It’s recommended that women start yearly mammograms at age 45.
- Potential Risks: Some women may face barriers that decrease the effectiveness of screenings.
Enhancements in Women’s Health Care
This new regulation is expected to address disparities in women's health care by promoting better access to necessary screenings. Increased awareness and improved protocols for mammograms can lead to higher compliance rates, positively impacting patient outcomes.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.