Lebrikizumab Receives FDA Approval for Atopic Dermatitis Management
FDA Approval for Lebrikizumab
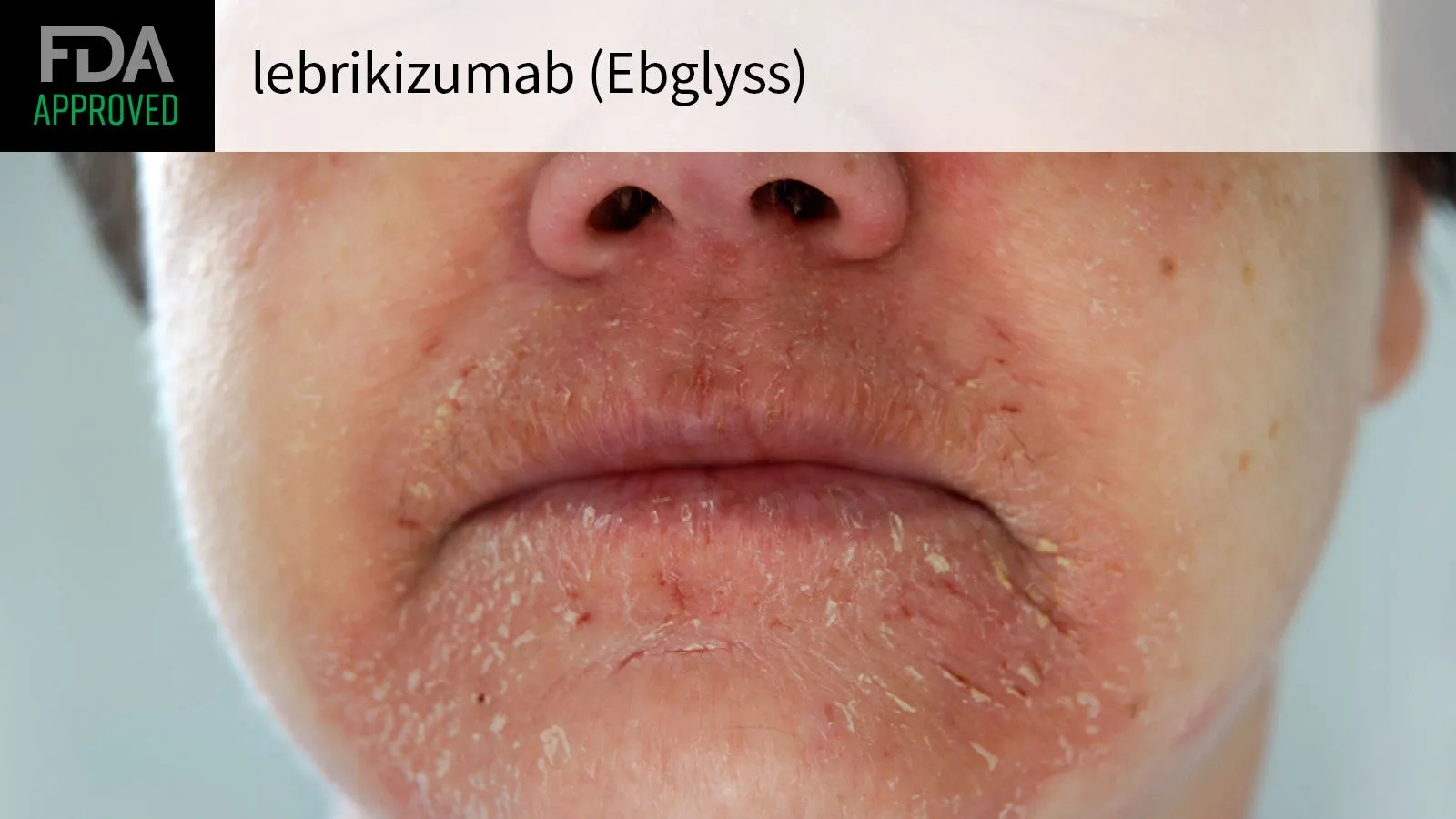
The FDA has officially approved lebrikizumab (marketed as Ebglyss) for the treatment of atopic dermatitis, commonly known as eczema, in both **adults** and **children** aged 12 and older. This class of treatment represents a significant stride in managing this prevalent chronic skin condition.
Implications of Lebrikizumab's Approval
Lebrikizumab targets the IL-13 cytokine, an important contributor to the pathogenesis of eczema. Its approval is expected to improve quality of life for many who struggle with the symptoms of dermatitis.
Key Benefits of Lebrikizumab
- Effective management of atopic dermatitis symptoms.
- Suitable for use in adolescents aged 12 and above.
- Helps reduce the need for oral corticosteroids.
Next Steps in Treatment Paradigm
Patients should consult their healthcare provider for tailored treatment plans, while physicians will need to evaluate the specific needs of their patients to decide on the best course of action.
For more information and the latest updates, visit the source.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.