WHO Prequalifies Vaccine Against Mpox: A New Era in Public Health
Understanding the Significance of the WHO's Prequalification
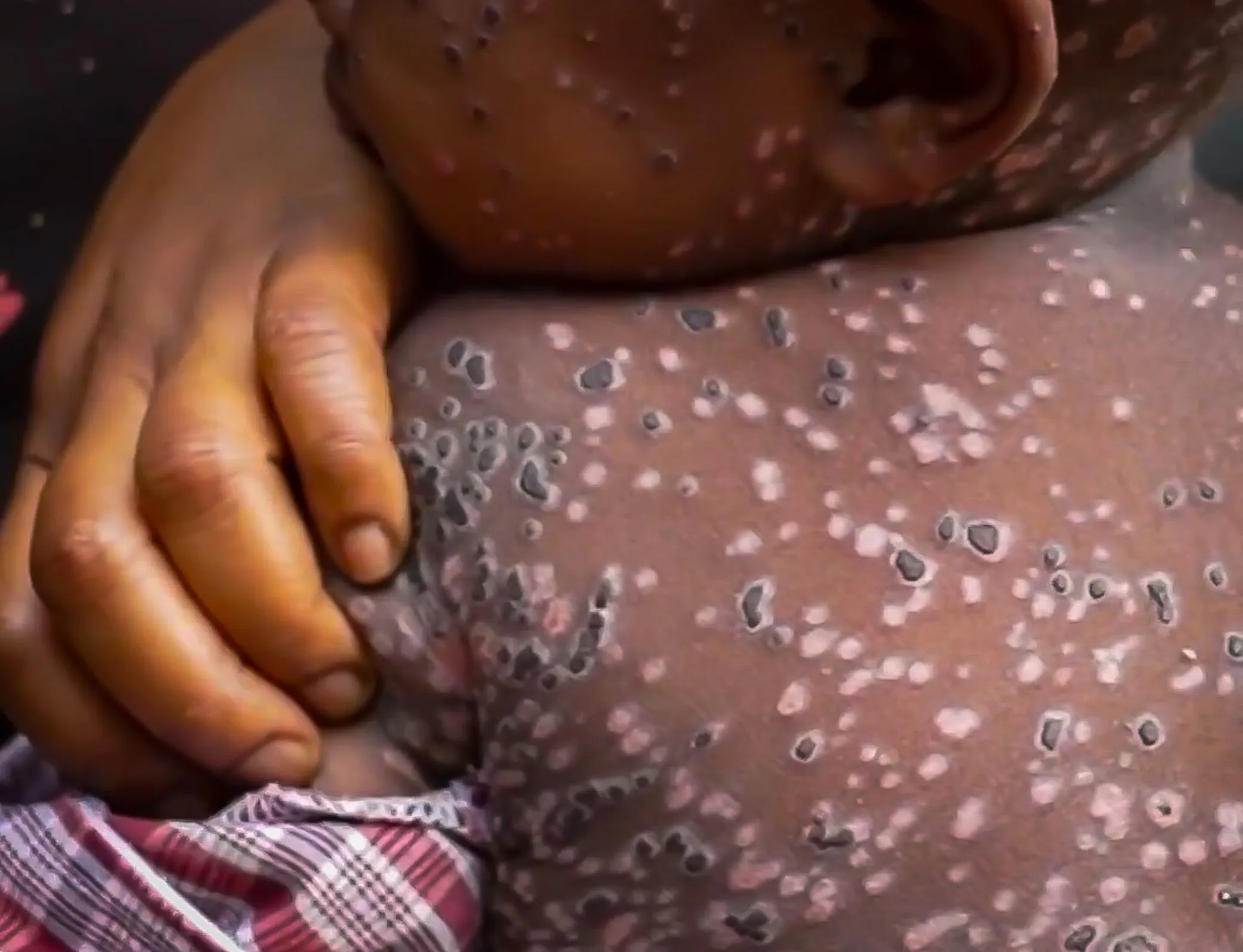
The World Health Organization (WHO) has made a groundbreaking decision in public health by granting prequalification to the MVA-BN vaccine. This vaccine is noteworthy as it is the first vaccine approved for mpox, an infectious disease that has raised global health concerns.
Key Features of the MVA-BN Vaccine
- Designed to combat mpox
- Enhances the global vaccination strategy
- Safety and efficacy profiles evaluated
With the WHO's prequalification, the MVA-BN vaccine can now be utilized in various health settings, emphasizing the importance of preparedness against future outbreaks. This approval not only instills hope but also prepares us to face new viral threats more effectively.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.