Medicine Research News: Advancements in the Lassa Fever Vaccine Development
Health Research Update: Lassa Fever Vaccine
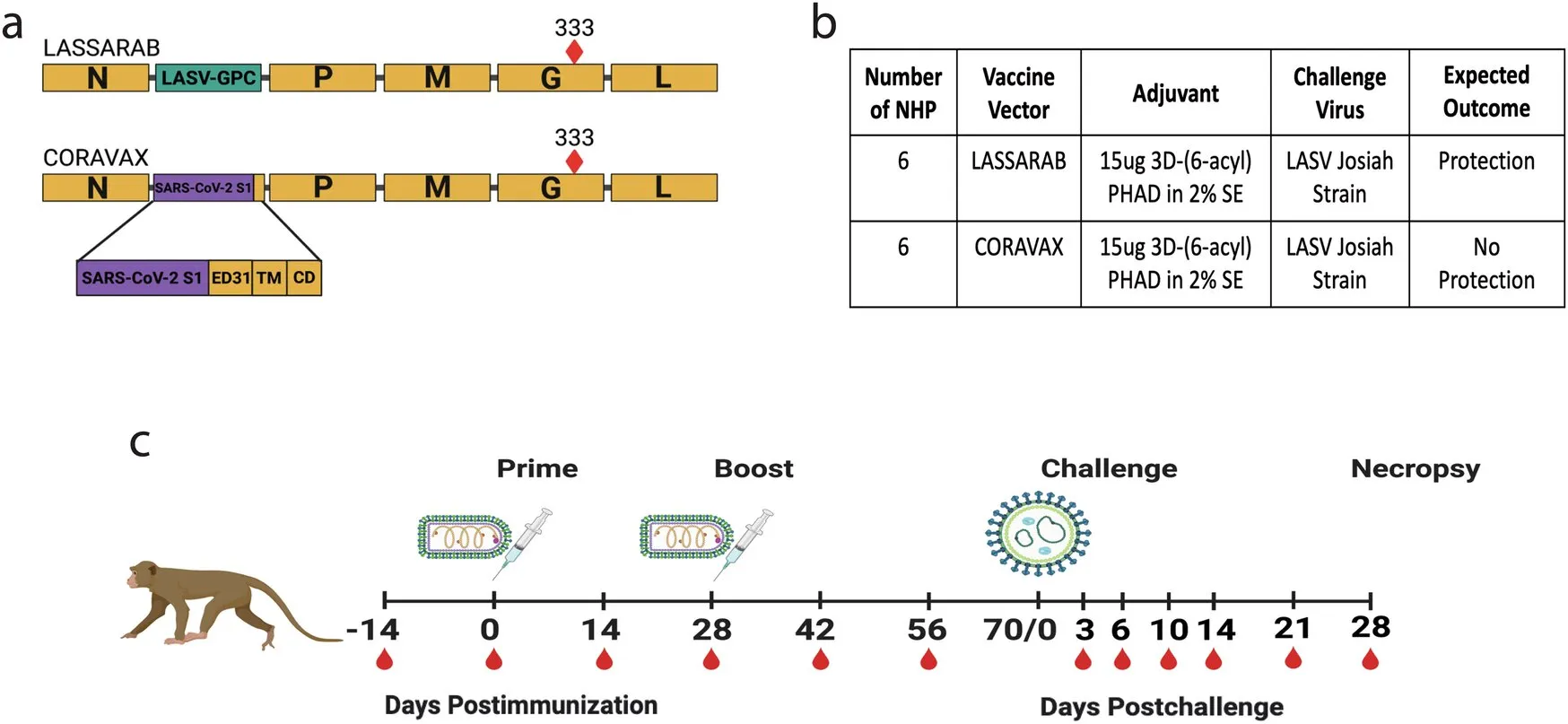
Health research reveals that a novel Lassa fever vaccine is set to enter Phase I clinical trials. This development has been driven by collaborative efforts between Thomas Jefferson University and the University of Maryland Baltimore, alongside the United States Army Medical Research Institute of Infectious Diseases (USAMRIID).
Key Milestones in Medicine Science
- Phase I Trials: The initial clinical phases will evaluate safety and immunogenicity.
- Collaborative Efforts: Integrating resources and expertise from multiple institutions strengthens the research.
- Public Health Impact: Successful vaccine development could lead to significant reductions in Lassa fever cases, enhancing community health.
Future Directions in Health Science
As this vaccine progresses, it may open new pathways for addressing similar viral threats, marking a pivotal moment in health research and prevention strategies.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.