Abortion Pill Regulation in Louisiana Impacts Women's Health
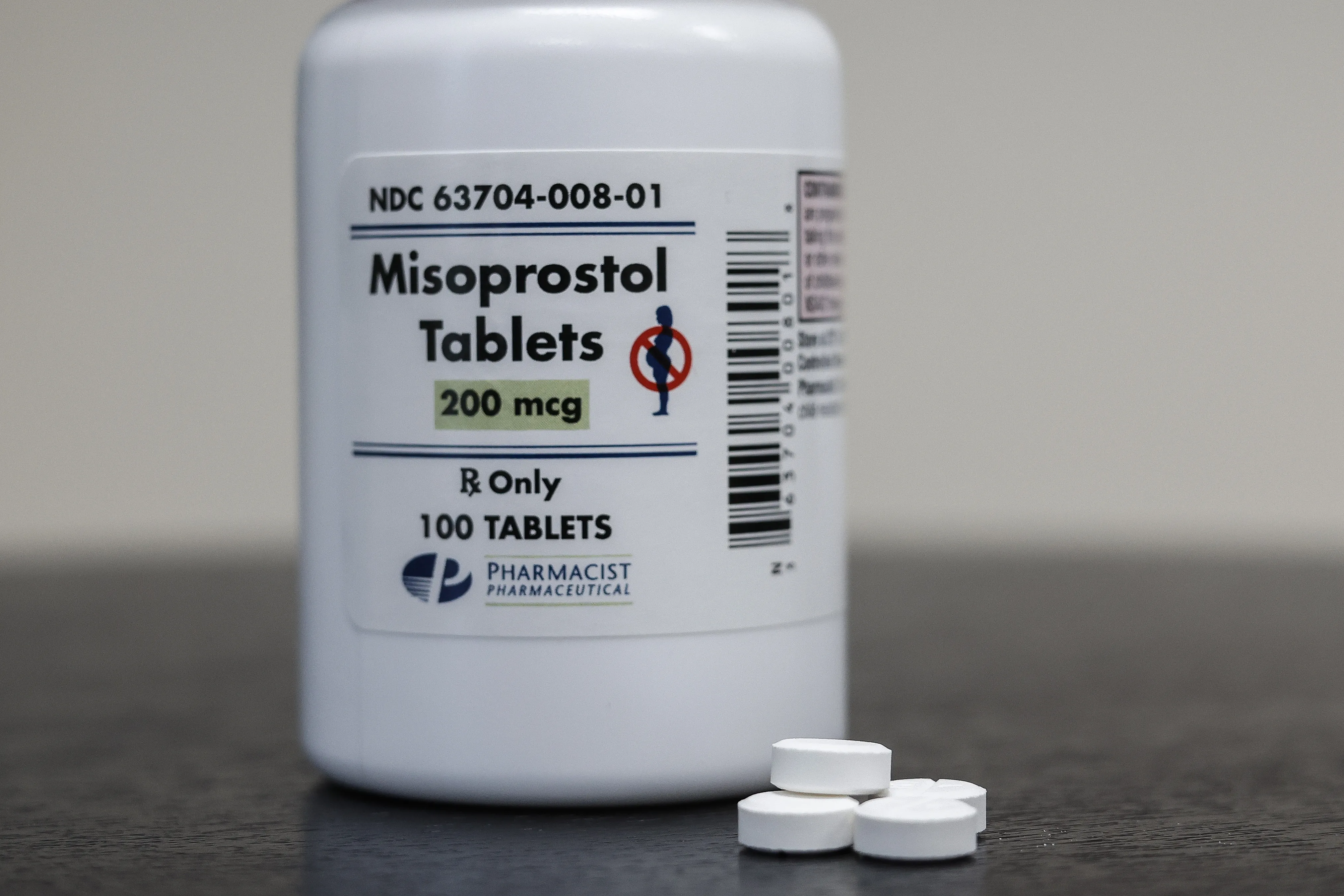
Changes in Louisiana's Abortion Pill Law
Recently enacted legislation in Louisiana has classified mifepristone and misoprostol as Schedule IV controlled substances. This change has raised alarms among health professionals.
Impact on Women's Health
Doctors assert that these regulatory adjustments could hinder access to essential care services for women. The designation of these medications as controlled substances may result in necessary delays and additional barriers to access.
What's at Stake?
- Timeliness of medical interventions
- Access to critical reproductive healthcare
- Increased patient anxiety and frustration
Potential Consequences
- Complications arising from delayed treatment
- Greater strain on healthcare providers
- Questions regarding legal compliance for practitioners
Disclaimer: The information provided on this site is for informational purposes only and is not intended as medical advice. We are not responsible for any actions taken based on the content of this site. Always consult a qualified healthcare provider for medical advice, diagnosis, and treatment. We source our news from reputable sources and provide links to the original articles. We do not endorse or assume responsibility for the accuracy of the information contained in external sources.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.