Covid-19 Trial Raises Ethical Concerns Over Moderna's £1,500 Offer to Children
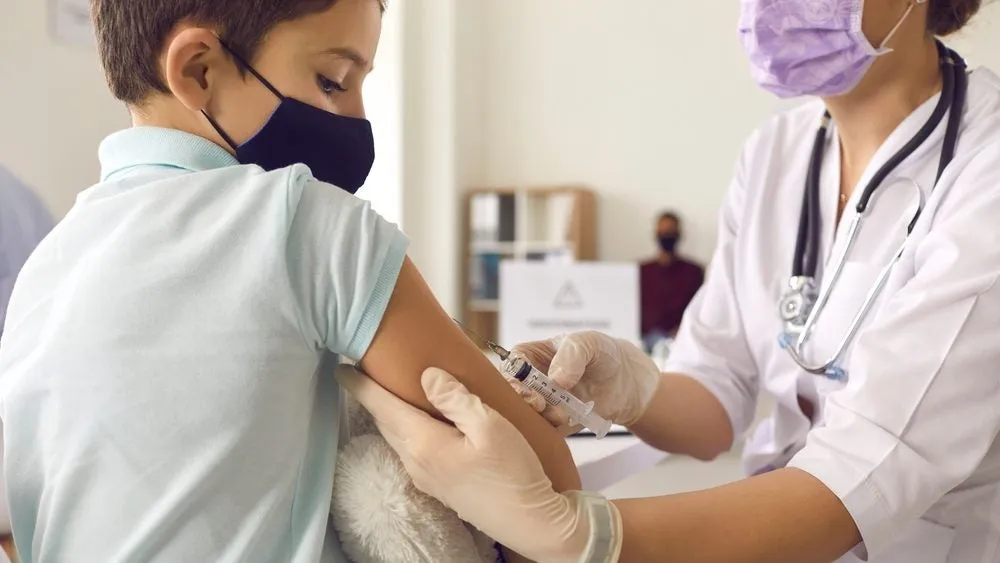
Ethical Dilemmas in Covid-19 Trials
The recent incident where Moderna, a leading vaccine manufacturer, offered £1,500 to children for participation in a Covid-19 trial has sparked significant controversy. The WhatsApp message sent by an NHS pediatrician raised concerns about the motivation behind such financial incentives in clinical research.
Key Concerns About Participation and Safety
- Financial incentives may exploit vulnerable families.
- Potential impact on children’s consent and autonomy.
- Heightened scrutiny on how Covid-19 trials are conducted.
As the health community grapples with these issues, advocacy for children's safety in medical trials remains paramount. Discussions on the ethics of such practices are essential in maintaining trust in medical research.
Disclaimer: The information provided on this site is for informational purposes only and is not intended as medical advice. We are not responsible for any actions taken based on the content of this site. Always consult a qualified healthcare provider for medical advice, diagnosis, and treatment. We source our news from reputable sources and provide links to the original articles. We do not endorse or assume responsibility for the accuracy of the information contained in external sources.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.