Science in the Pharmaceutical Industry: Addressing Health Compliance Issues in France
Significant Sanctions in France's Pharmaceutical Sector
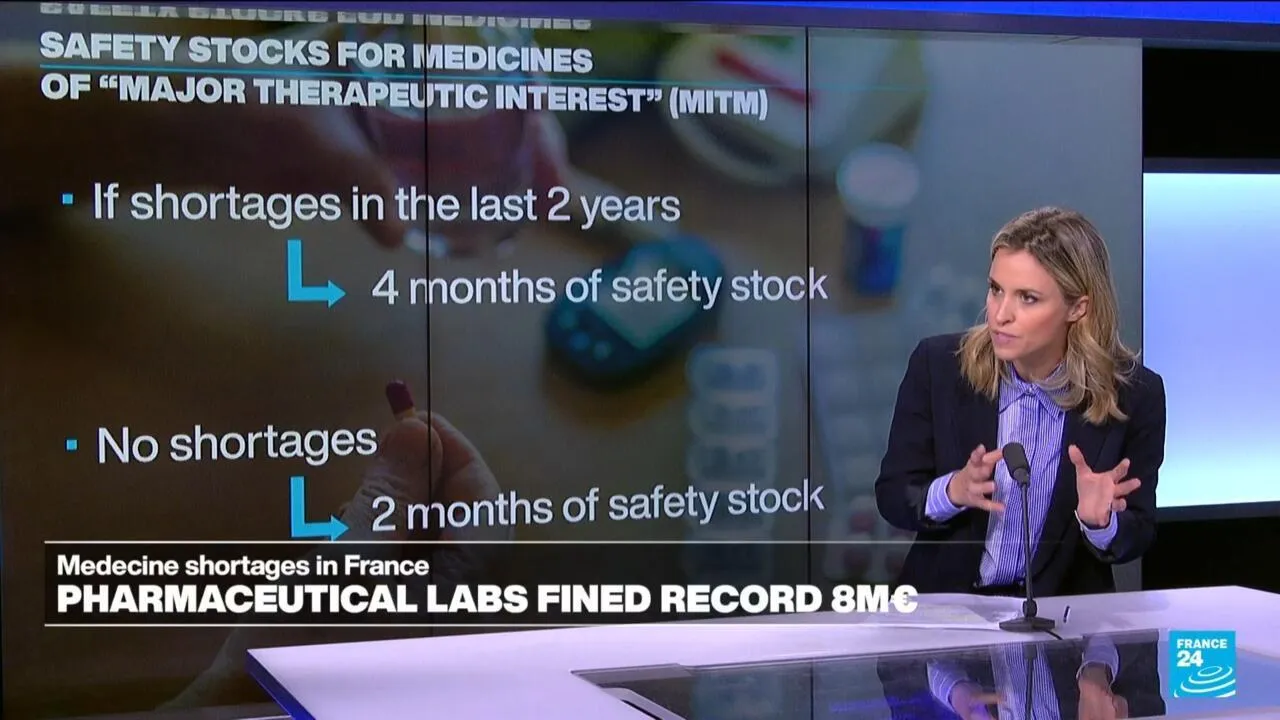
The French Medicines Agency has imposed significant fines on 11 pharmaceutical companies for violating stock regulations. These firms failed to maintain the required minimum of four-month supplies for essential medications of major therapeutic interest.
Implications for Health and Safety
This crackdown highlights the imperative for compliance within the pharmaceutical industry to safeguard public health. By enforcing these regulations, France aims to ensure that necessary medications remain available to patients.
- Increased oversight motivates compliance within the industry.
- Protecting public health is a top priority.
- Compliance can prevent future medication shortages.
Disclaimer: The information provided on this site is for informational purposes only and is not intended as medical advice. We are not responsible for any actions taken based on the content of this site. Always consult a qualified healthcare provider for medical advice, diagnosis, and treatment. We source our news from reputable sources and provide links to the original articles. We do not endorse or assume responsibility for the accuracy of the information contained in external sources.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.