FDA Approves First At-Home Flu Vaccine: FluMist Nasal Spray for All Ages 2-49
Revolutionizing Flu Prevention with Nasal Spray
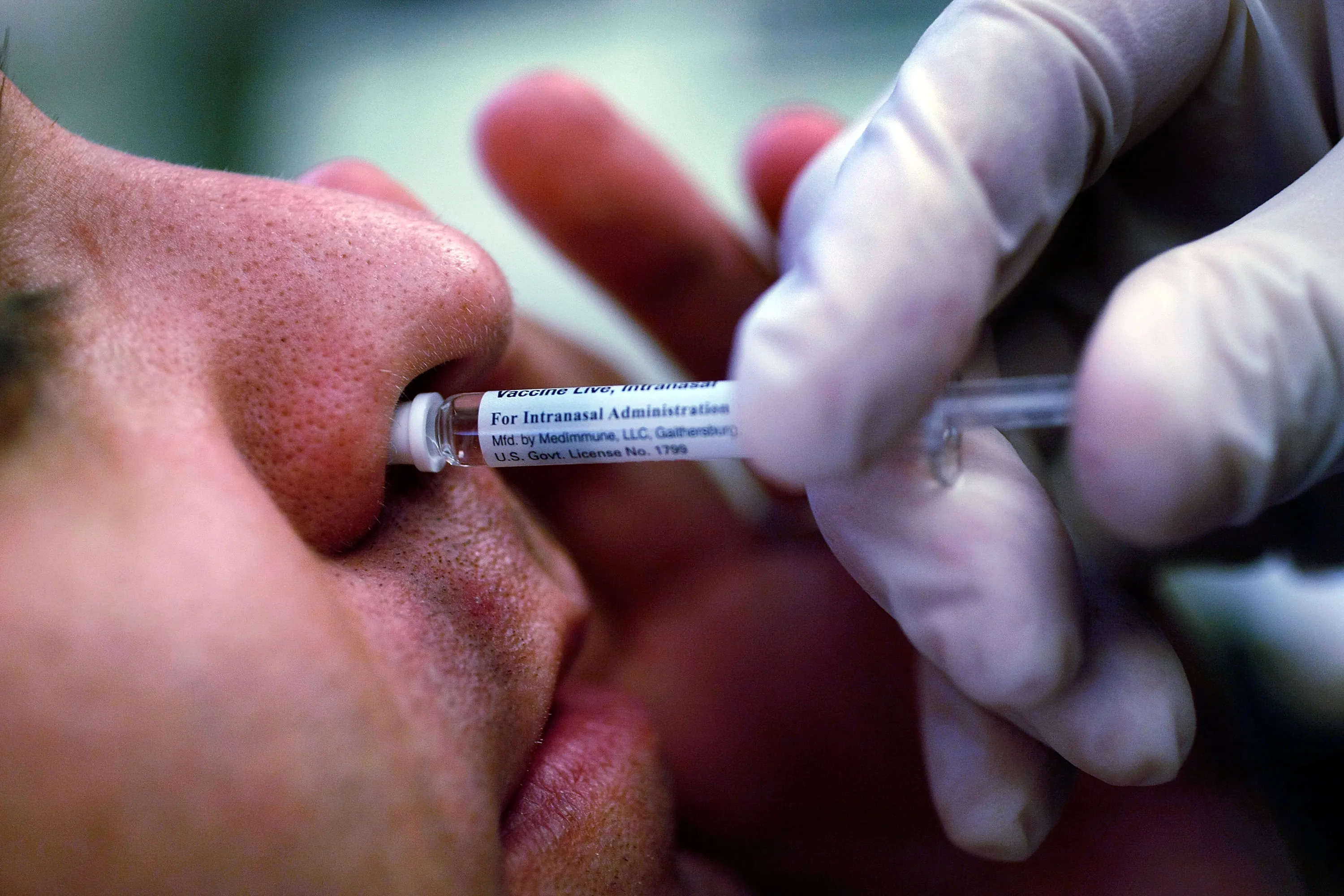
The FDA has recently approved FluMist, the first at-home flu vaccine available in the U.S. This nasal spray offers a non-invasive alternative to traditional flu shots, making vaccination easier and more accessible for people aged 2 to 49.
Benefits of FluMist
- Non-invasive delivery method
- Suitable for various age groups
- Enhances compliance in vaccination rates
With this approval, FluMist represents a hopeful option for many who prefer a spray over injections. Public health officials anticipate improved community immunity and a reduction in flu transmission.
Disclaimer: The information provided on this site is for informational purposes only and is not intended as medical advice. We are not responsible for any actions taken based on the content of this site. Always consult a qualified healthcare provider for medical advice, diagnosis, and treatment. We source our news from reputable sources and provide links to the original articles. We do not endorse or assume responsibility for the accuracy of the information contained in external sources.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.