FDA's Approval of Self-Administered Flu Vaccine Spray for Flu Season
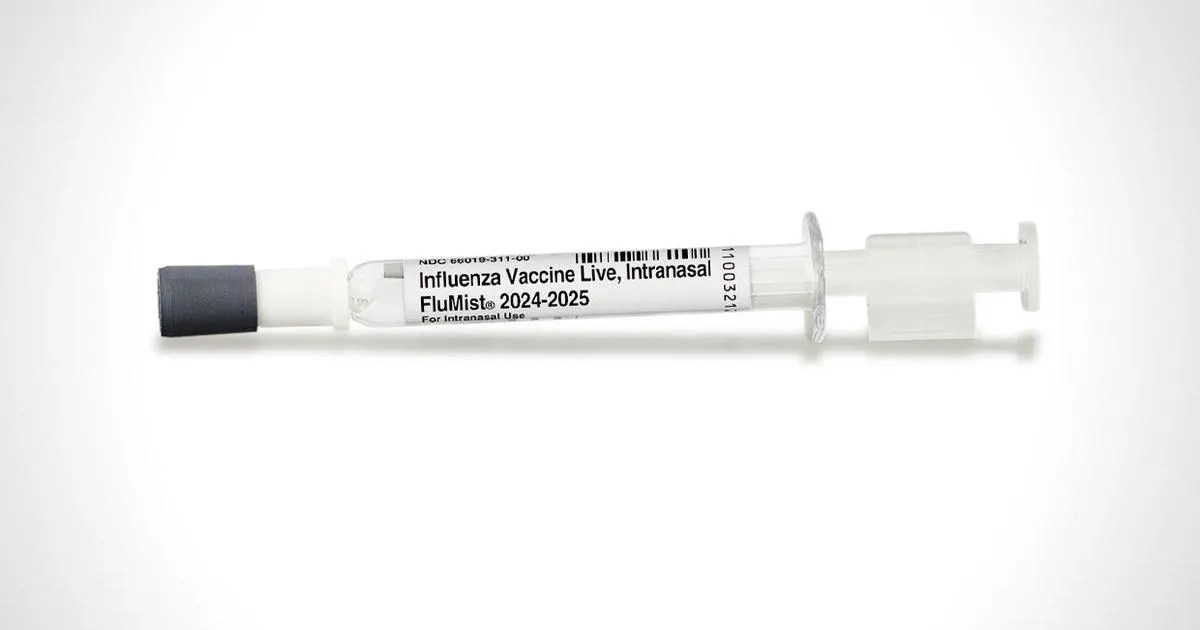
FDA Approves First Self-Administered Flu Vaccine Spray
The Food and Drug Administration (FDA) announced a significant advancement in flu prevention on Friday. They have broadened the approval of the FluMist nasal spray, making it the first self-administered flu vaccine spray available to the public.
Significance of the Approval for Flu Season
- Convenience: Patients can administer the spray themselves, enhancing accessibility.
- Effectiveness: This method may increase vaccination rates in communities.
- Public Health: The approval aims to reduce the incidence of flu during peak flu season.
Healthcare providers are encouraged to discuss this new option with their patients, promoting vaccination as a primary defense against seasonal flu.
Disclaimer: The information provided on this site is for informational purposes only and is not intended as medical advice. We are not responsible for any actions taken based on the content of this site. Always consult a qualified healthcare provider for medical advice, diagnosis, and treatment. We source our news from reputable sources and provide links to the original articles. We do not endorse or assume responsibility for the accuracy of the information contained in external sources.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.