FDA Advisory Panel Discusses Merck and Bristol Myers Cancer Drugs
Friday, 23 August 2024, 15:25
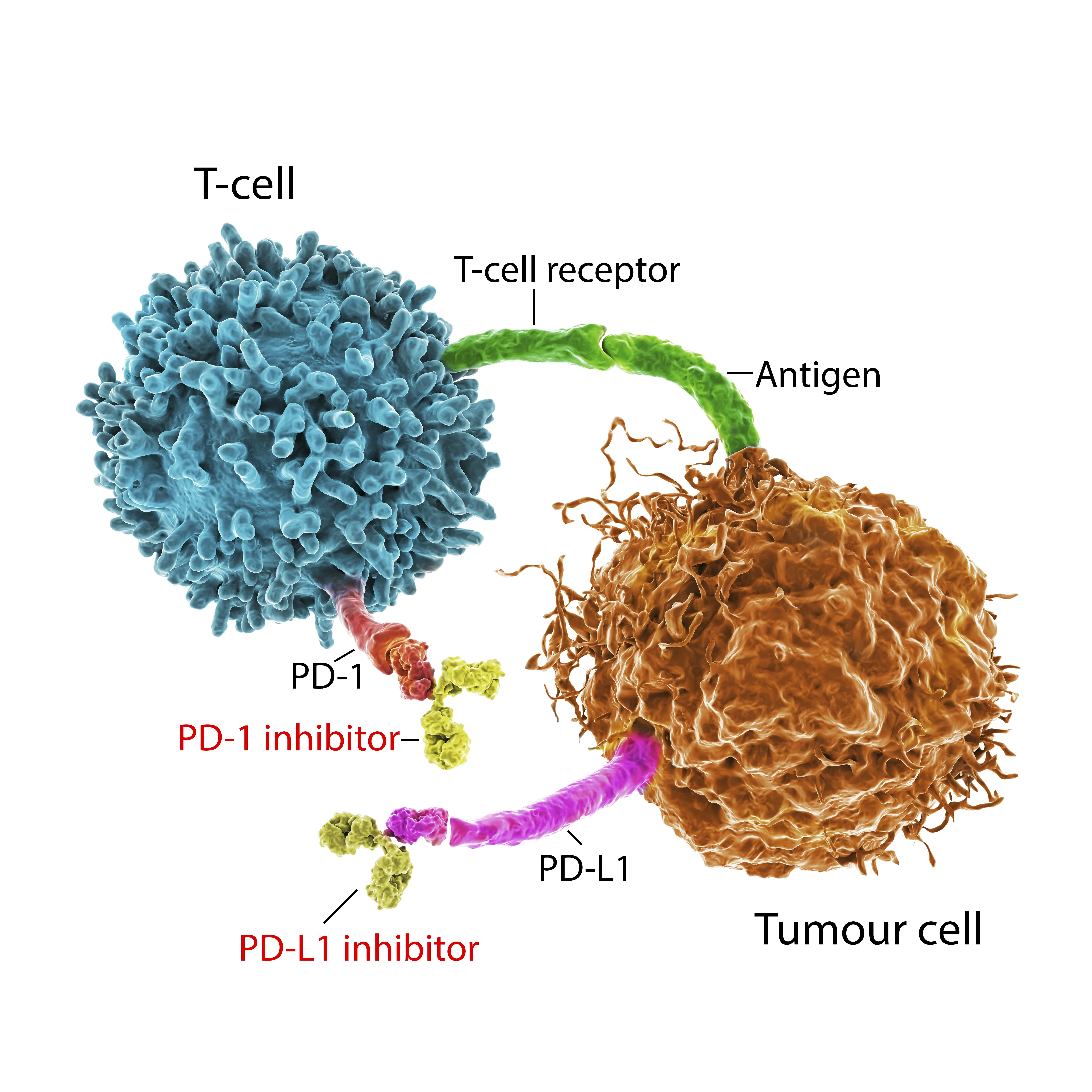
Key Insights on the FDA Advisory Panel Meeting
In September, the FDA advisory panel will review the usage of immune checkpoint inhibitors
- Merck and Bristol Myers' drugs under scrutiny
- Discussions focus on application changes for specific cancers
- Potential impacts on treatment protocols
Future Implications of This Meeting
As the FDA panel convenes to discuss these cancer treatment innovations, the implications for patient care and pharmaceutical strategies could be significant, impacting stock prices and healthcare planning.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.