FDA Advisory Panel on Merck and Bristol Myers Cancer Drugs: Key Insights
September FDA Advisory Panel Meeting Overview
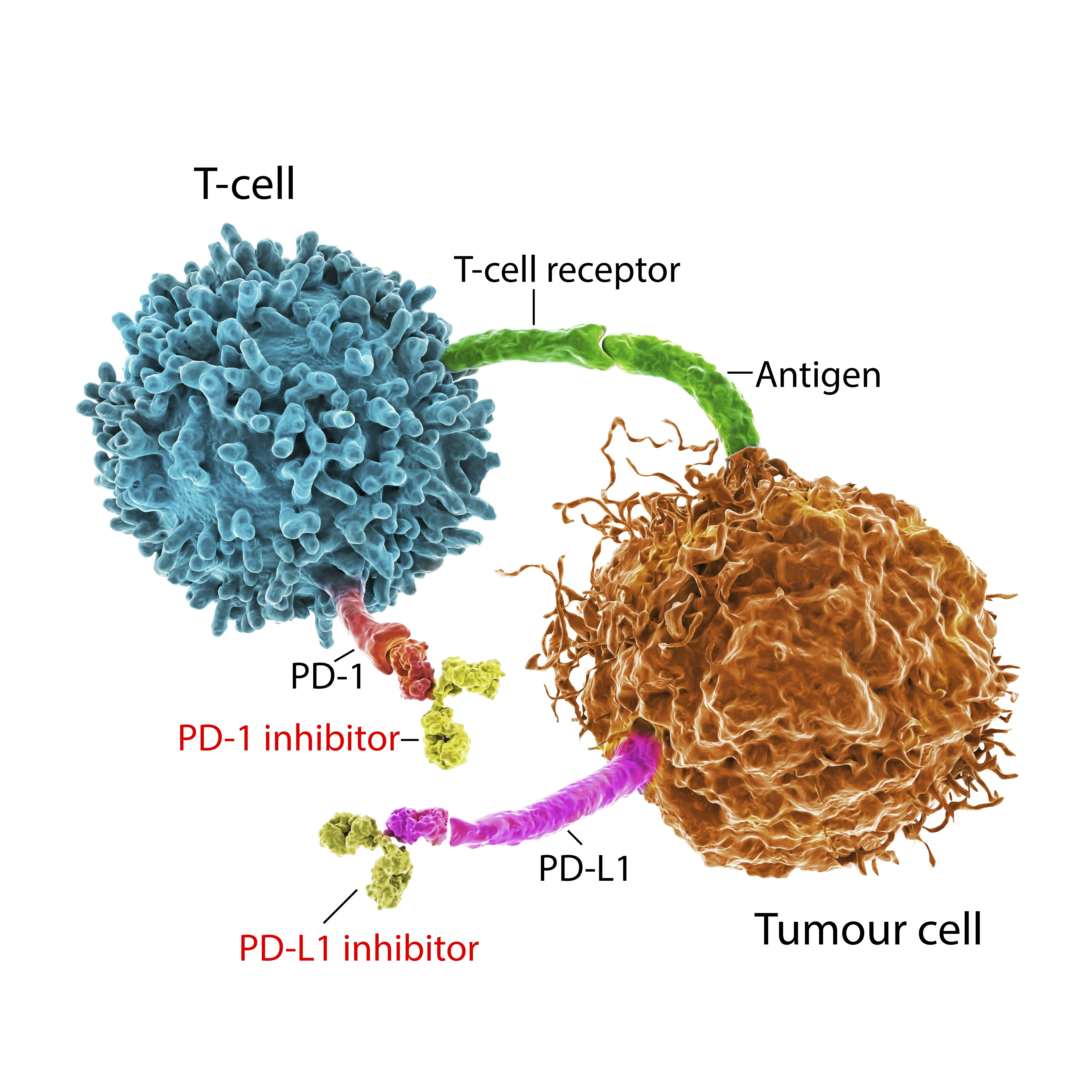
The FDA advisory panel meeting scheduled for September is aimed at evaluating the use of immune checkpoint inhibitors, particularly those produced by Merck and Bristol Myers. This critical evaluation could lead to new recommendations regarding their usage in various cancer treatments.
Implications for Cancer Treatment
The outcome of this meeting may significantly impact treatment protocols for cancer patients. Health practitioners and pharmaceutical companies express concerns about potential restrictions that the panel might propose.
- Potential restrictions on key drugs
- Impact on patient care
- Future of cancer treatment options
As discussions unfold, it will be essential for stakeholders to stay informed regarding the panel's recommendations and their implications for ongoing treatments.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.