FDA's Historic Approval of Neffy: A Breakthrough in Allergy Treatment
Introduction
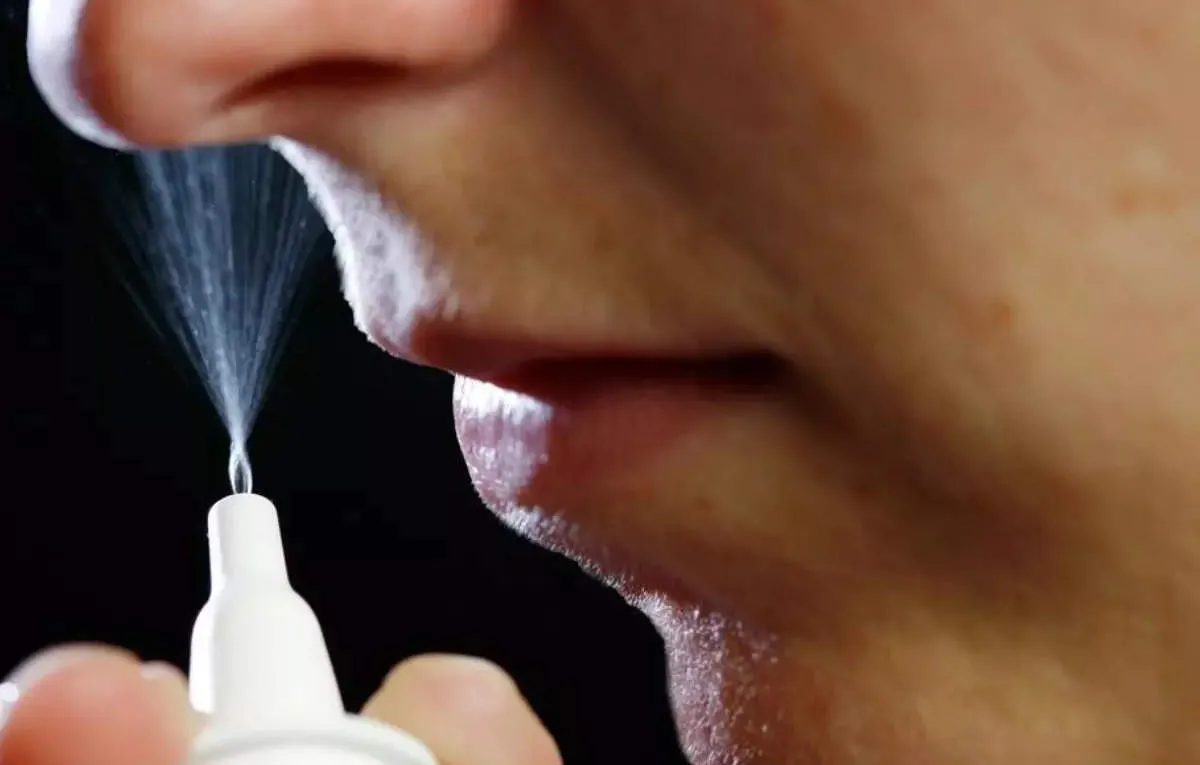
The FDA has officially approved Neffy, a groundbreaking nasal spray aimed at treating allergic reactions.
Clinical Studies
- The approval was based on four studies.
- 175 healthy adults participated without anaphylaxis.
- This validated the efficacy of epinephrine concentrations.
Significance
This approval introduces a novel treatment option for those suffering from severe allergies, making it easier to manage symptoms.
Conclusion
Neffy serves as a crucial advancement in the realm of allergy treatment, providing patients with a convenient alternative to epinephrine injections, enhancing their ability to respond to allergic emergencies.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.