FDA's New Nasal Spray Offers Promising Alternative for Anaphylaxis Treatment
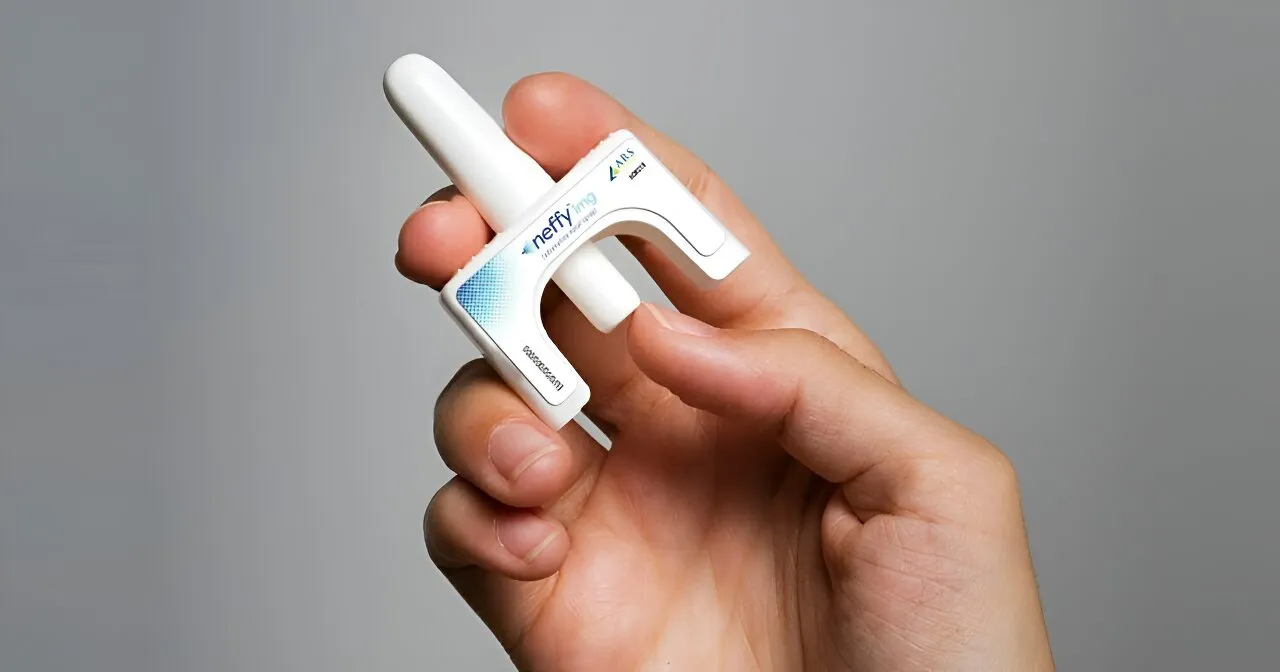
FDA Approval of Nasal Spray
The FDA has made a landmark decision by granting approval for the first nasal spray aimed at curbing anaphylaxis. This development comes as a relief for those who are apprehensive about administration methods traditionally relying on injections.
Benefits of the Nasal Spray
- Alternative Treatment: Provides a distressing-free option for individuals.
- Accessibility: Increases the ease of use during emergency situations.
- Confidence: Empowers patients with a user-friendly alternative.
Conclusion
This approval highlights a significant advancement in anaphylaxis treatment, aiming to improve the lives of those affected. Patients can now explore a more suitable option that meets their needs, ultimately affecting how severe allergic reactions are managed.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.