Promising Advances in Colorectal Cancer Screening: The Shield Test
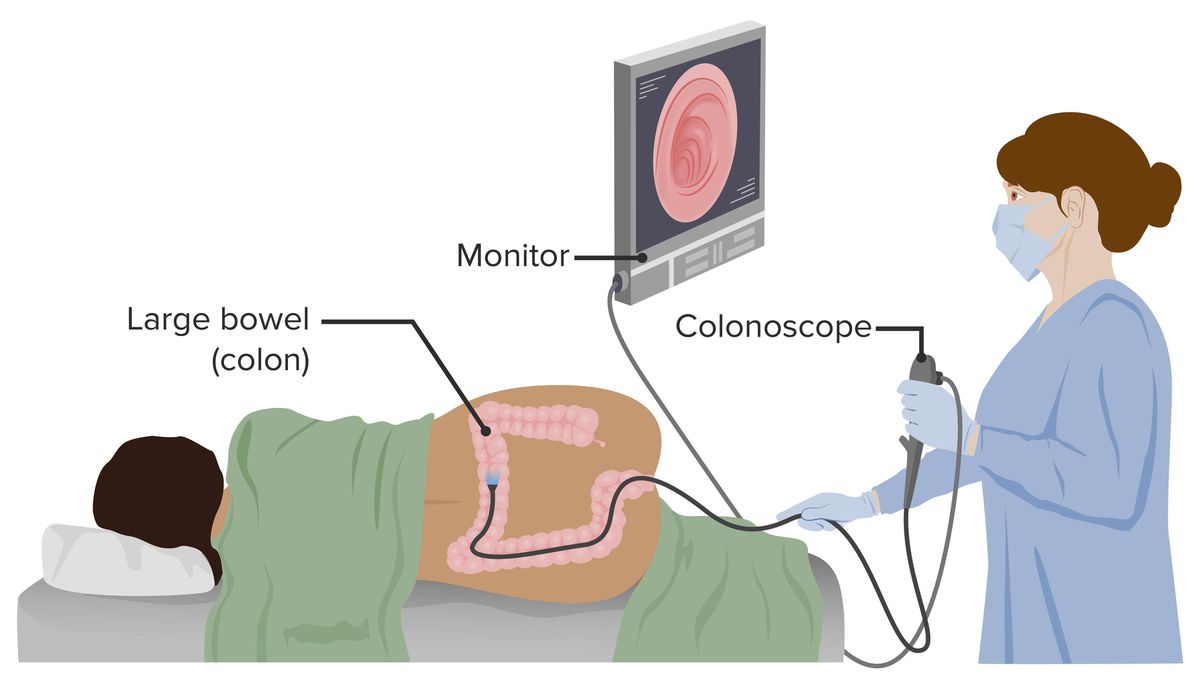
Overview of the New Shield Test
The Shield test, a novel blood test for colorectal cancer, is generating significant excitement among healthcare professionals.
FDA Approval Process
The test is currently progressing towards FDA approval, which could revolutionize the detection of colorectal cancer.
Benefits Over Traditional Methods
- Less invasive than colonoscopies
- Potential for earlier detection
- Improved patient accessibility
Conclusion
If approved, the Shield test may significantly improve early detection rates of colorectal cancer, offering hope for better outcomes in patients.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.