Previewing the FDA Decision for Eyenovia's APP13007
Thursday, 29 February 2024, 17:02
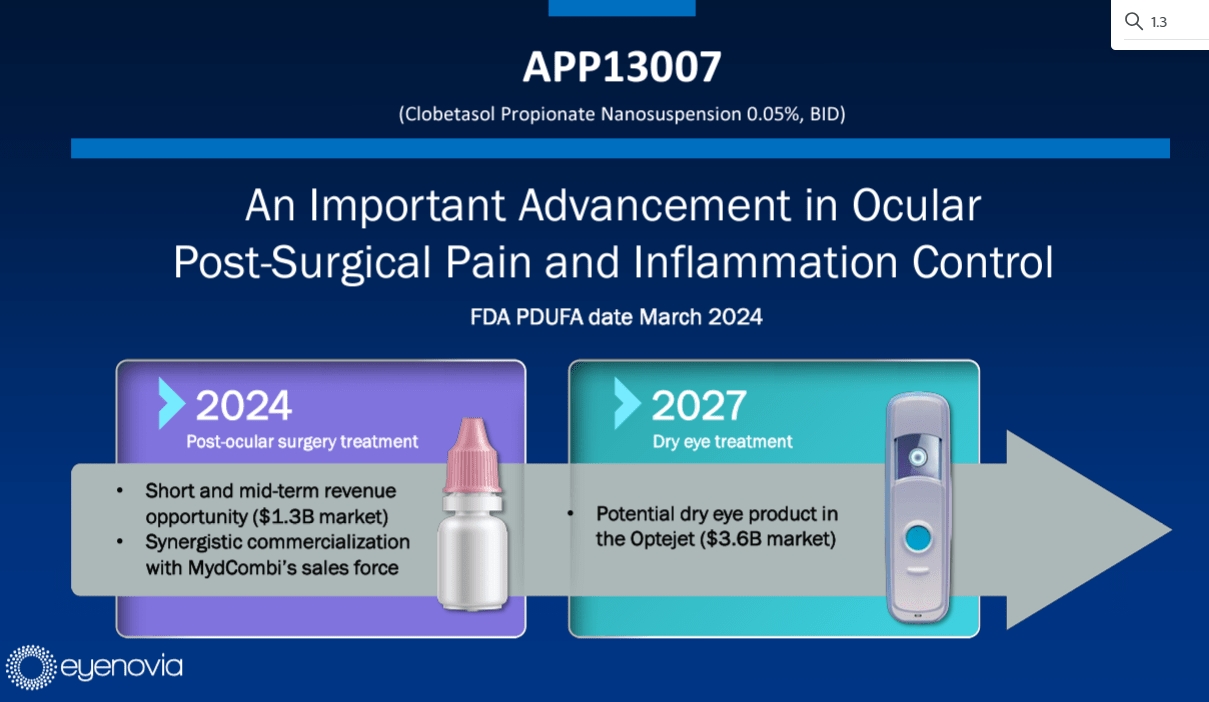
Eyenovia's APP13007 FDA Decision Preview
Eyenovia's (EYEN) APP13007 is scheduled for an FDA decision next week. This approval could be a game-changer for the company and investors.
Key Points:
- Important FDA Decision: The fate of Eyenovia's drug will be decided.
- Market Impact: Approval could lead to a surge in share price.
- Investors are eagerly awaiting the outcome.
Stay tuned for the latest updates on Eyenovia and FDA decisions.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.