Novo Nordisk's Plea to FDA: Ban on Compounding Pharmacies for Ozempic, Wegovy Copies
Challenges in Biotech: Novo Nordisk's Position
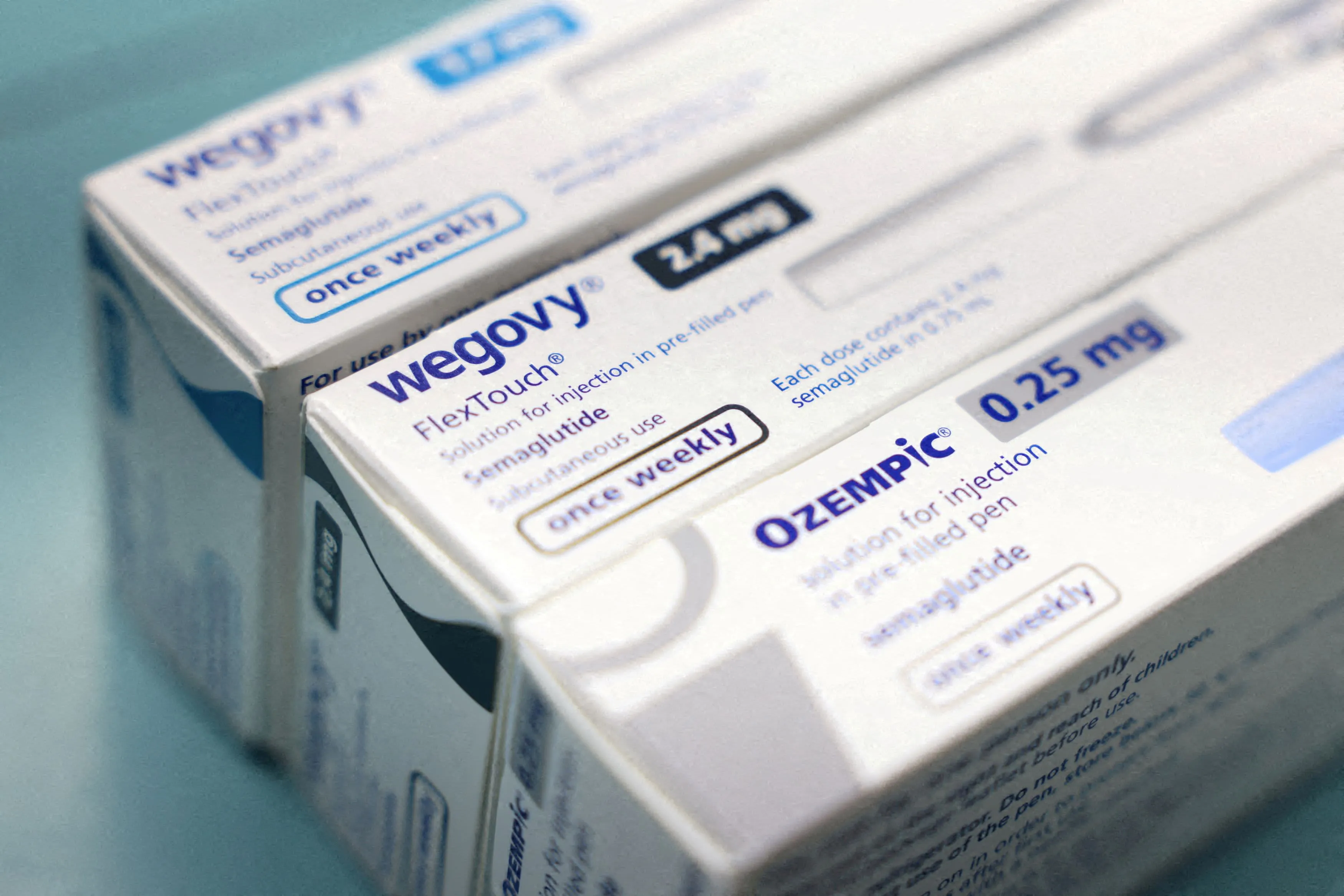
Novo Nordisk A/S, a leading name in the health care industry, has formally requested the FDA to restrict compounding pharmacies from producing their own versions of Ozempic and Wegovy. The active ingredient, semaglutide, poses intricate manufacturing challenges that, according to the company, could jeopardize patient safety.
Implications for Pharmaceuticals
The implications of this request extend beyond Novo Nordisk, as it highlights broader issues within the biotechnology and pharmaceuticals sectors. bIf successful, it may reshape the landscape of the health care industry and manage market competition effectively.
- Semaglutide's Complexity
- Safeguarding Patient Safety
- The Role of Compounding Pharmacies
Market Response and Future Developments
Should the FDA grant this request, i investors could see significant shifts in stock performance for companies involved in the biotechnology segment. Also, it may lead to a surge in demand for Novo Nordisk’s products.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.