Ocugen Stock Gains FDA Approval for Phase 3 Gene Therapy Trial
Monday, 8 April 2024, 11:10
Ocugen Stock Gains FDA Approval for Phase 3 Gene Therapy Trial
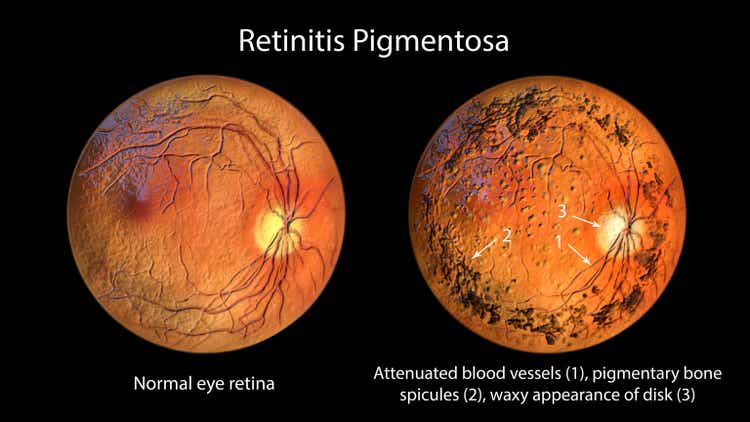
Ocugen experiences a notable surge in stock price following the FDA's clearance for the initiation of a Phase 3 clinical trial for their gene therapy candidate focused on retinitis pigmentosa treatment.
Key Highlights:
- Significant Milestone: FDA approves Ocugen's gene therapy clinical trial development.
- BLA Objective: Company aims for Biologics License Application approval by 2026.
The approval marks a promising step towards addressing retinitis pigmentosa, a genetic eye disorder with limited treatment options.
This article was prepared using information from open sources in accordance with the principles of Ethical Policy. The editorial team is not responsible for absolute accuracy, as it relies on data from the sources referenced.